
No stopping Astra’s pivotal TIGIT plan

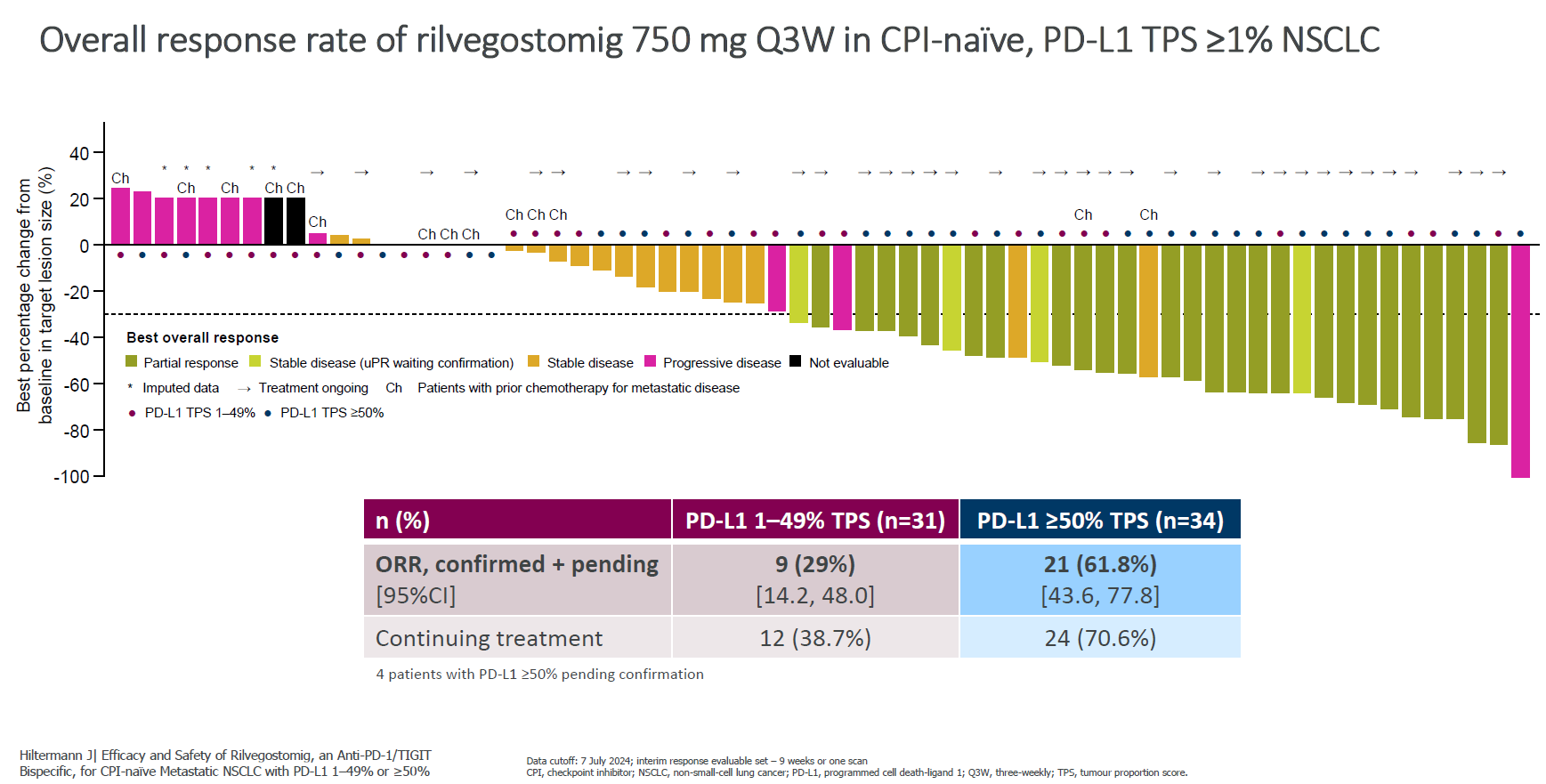
Shortly after revealing first-in-human lung cancer results for rilvegostomig at the World Conference on Lung Cancer, AstraZeneca is starting another NSCLC trial of this anti-TIGIT x PD-1 MAb. However, the pivotal Artemide-Lung03 study tests rilvegostomig plus chemo in first-line non-squamous NSCLC, whereas the phase 1/2 Artemide-01, the subject of the World Lung presentation, concerned rilvegostomig monotherapy in checkpoint-naive patients irrespective of histology who could have received prior chemo. Both, however, limit recruitment to PD-L1-expressers – surprising for Artemide-Lung03 given that this is a chemo combo trial. The phase 3 will compare the combo against Keytruda plus chemo on co-primary endpoints of OS and PFS, the baseline being the roughly 24-month median OS shown by Keytruda plus chemo in the PD-L1-expressing subgroup of Keynote-189. At World Lung rilvegostomig activity correlated with PD-L1 expression, with a 750mg three-weekly dose hitting a 62% ORR (including unconfirmed responses) in PD-L1 ≥50% expressers. Paradoxically 1.5g yielded just 37% in the same subgroup, and caused higher rates of low-grade adverse events including liver toxicity, and 750mg was chosen as the pivotal dose. Astra is also running the phase 3 Tropion-Lung10 trial in first-line PD-L1 ≥50% non-squamous NSCLC, comparing datopotamab deruxtecan plus rilvegostomig versus rilvegostomig monotherapy.
Rilvegostomig in Artemide-01
1983













