Multiple myeloma Car-Ts face a US grilling
The FDA highlights high death rates and questions overall survival for Carvykti and Abecma.
The FDA highlights high death rates and questions overall survival for Carvykti and Abecma.

Until now the prevailing wisdom has been that, in the battle of the anti-BCMA Car-Ts, Johnson & Johnson/Legend’s Carvykti was superior to Bristol Myers Squibb/2seventy’s Abecma. However, FDA briefing documents released today ahead of an adcom on Friday suggest that the products are more alike than different where it really matters: clinical data.
Both therapies are already marketed for late-line multiple myeloma, and now their sponsors are gunning for approvals in earlier settings. But the FDA has flagged concerns about a high rate of early deaths seen in both products’ pivotal studies, Cartitude-4 for Carvykti and Karmma-3 for Abecma.
As a side note, the FDA also noted that it its intention to hold an adcom for Carvykti had been communicated to the sponsors on 8 December, via teleconference. Legend, which earlier said it didn't expect an adcom, didn't disclose that there would be one after all until late January 2024.
Overall survival questions
Both trials met their primary endpoints of progression-free survival – Carvykti’s more convincingly than Abecma’s. But the FDA today made clear its need for supplementary overall survival data. And it's here that the Car-T’s problems begin.
It was already known that Karmma-3 hadn't shown a benefit on OS, and today’s briefing documents highlighted the fact that the survival curves crossed over at around 15 months, showing a subset of patients who received Abecma dying more quickly.
The surprise was that a similar trend was seen in the Cartitude-4 results, with those survival curves crossing over at around 11 months. While Carvykti showed a benefit after 11 months, the FDA noted that these data were difficult to interpret because of “heavy censoring” later on in the survival curves.
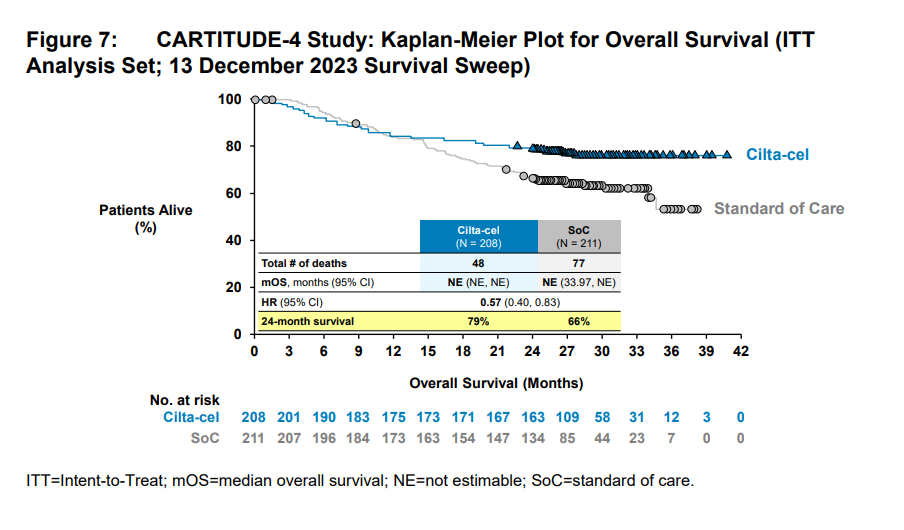
The FDA noted that the pattern of early deaths alongside a PFS benefit could be down to a the inclusion of a subgroup of patients for whom Carvykti therapy might be inappropriate.
On Friday the panel will weigh in on the specific question of whether "the risk of early death linked with [both Abecma and Carvykti] is acceptable". If it decides that it isn't then these products look likely to be confined to a multiple myeloma niche.
1324