
Lilly calls time on IDH1/2 inhibition
Servier's vorasidenib had shown promise at ASCO, but Lilly's dual IDH project LY3410738 leaves the pipeline.
Servier's vorasidenib had shown promise at ASCO, but Lilly's dual IDH project LY3410738 leaves the pipeline.

The industry pipeline is full of inhibitors of either IDH1 or IDH2, but molecules hitting both are few and far between. This week Lilly disclosed that its own dual incumbent, LY3410738, was being removed from its pipeline.
The company had claimed that, unlike reversible inhibitors, LY3410738's unique covalent binding in the allosteric pocket of mutant forms of IDH1 gave it the ability to maintain potency in the setting of acquired resistance mutations seen in response to IDH1 and IDH2 inhibitors. However, first-in-human data presented at AACR 2023 showed that efficacy appeared limited in IDH inhibitor-pretreated patients.
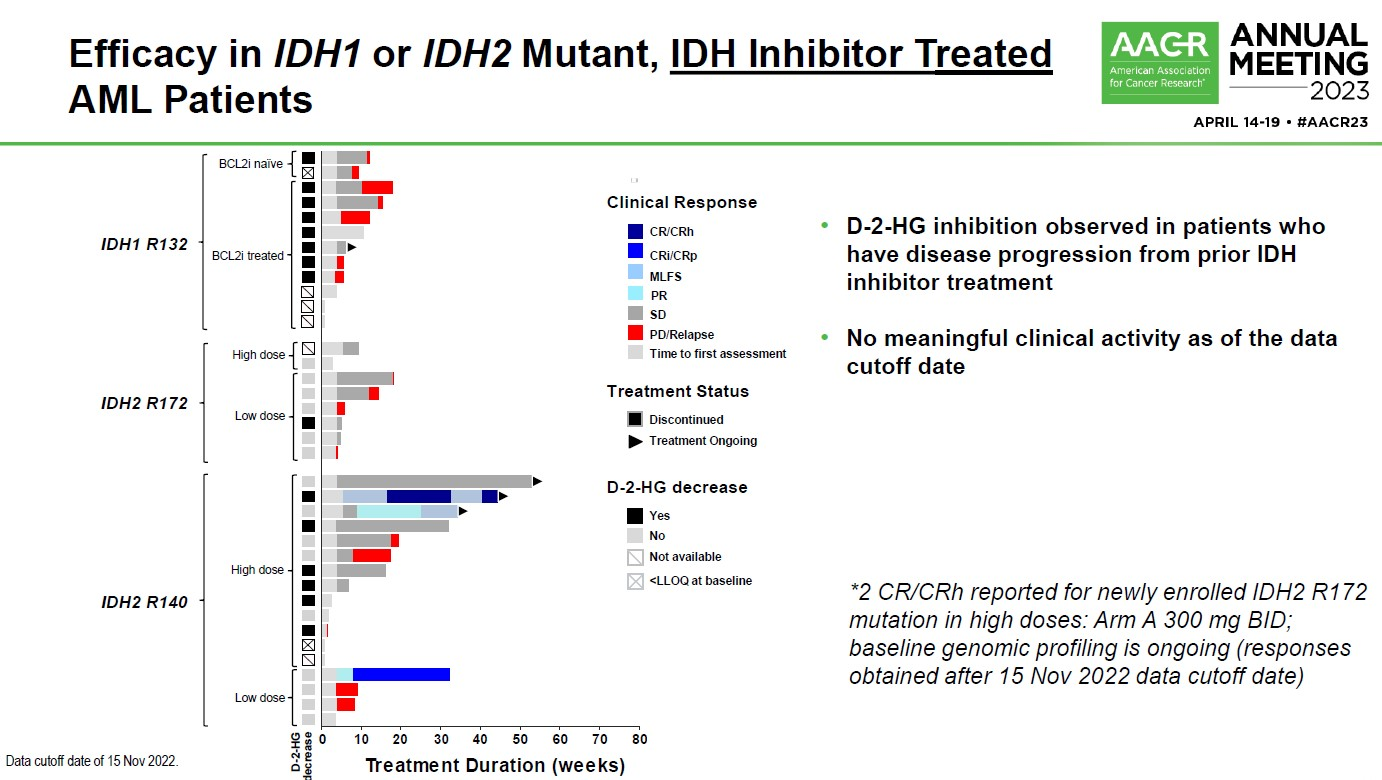
At the time Lilly said this might be indicative of "non-IDH dependent mechanisms in these patients", but ultimately it seems that whatever problems existed could not be ironed out. LY3410738, an asset acquired through Lilly's purchase of Loxo, appears in three phase 1 studies, one of which has been marked "terminated" on clinicaltrials.gov.
The discontinuation comes after Servier presented phase 3 data at ASCO on its pan-IDH inhibitor vorasidenib, acquired from Agios, showing impressive activity in low-grade glioma, albeit at the expense of liver toxicity, which resulted in two cases of Hy's law. Despite the side effects, doctors expressed optimism at vorasidenib's potential to become the first targeted therapy for this type of cancer.
It does not appear that Lilly had experienced meaningful toxicity problems with LY3410738; its AACR presentation stated that the safety profile was favourable, with no QTc prolongation or liver toxicities observed.
Selected IDH1/2 inhibitors
| Generic name | Company | Status |
|---|---|---|
| Vorasidenib | Servier (ex Agios) | 8 trials, 593 patients |
| HMPL-306 | Hutchmed | 3 trials, 240 patients |
| NMS-03602173 | Nerviano | US clinical trial cleared in Oct 2022, but no studies evident |
| LY3410738 | Lilly | Discontinued |
Source: OncologyPipeline.
745













