iTeos’s TIGIT goes pivotal at last

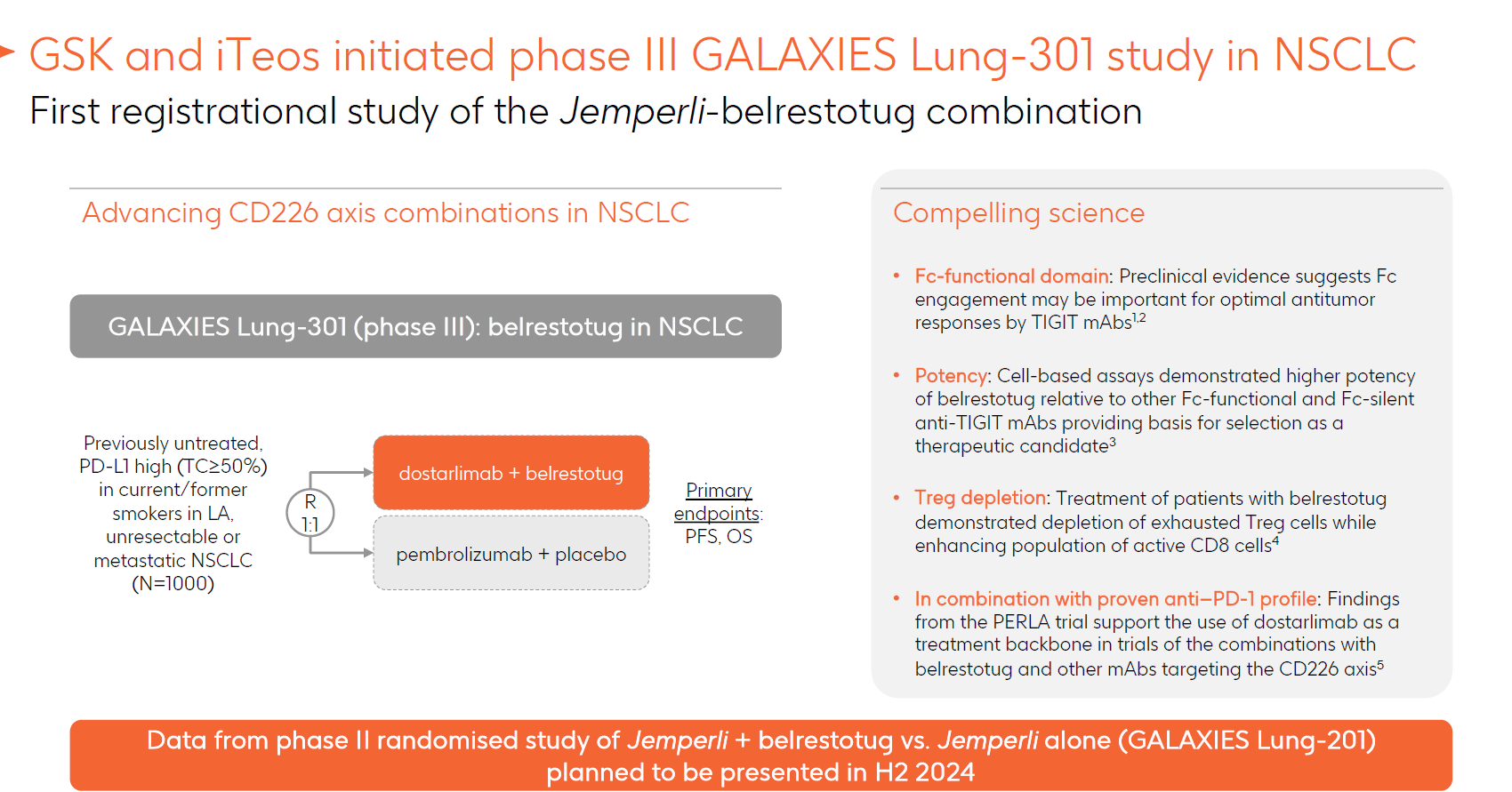
Any iTeos investors hoping for a glimpse of how their company’s anti-TIGIT MAb belrestotug performed in its delayed Galaxies Lung-201 study in first-line NSCLC will have to wait a little longer. GSK's oncology event this week simply repeated that the data, toplined as showing belrestotug to have met “predefined efficacy criteria”, would be presented in the second half. What was highlighted, however, was that GSK had launched a phase 3 belrestotug study, Galaxies Lung-301, pitting a Jemperli combo head to head against Keytruda in 1,000 first-line NSCLC patients whose cancers express PD-L1 at 50% or higher, and testing OS and PFS as co-primary endpoints. That study has yet to be registered on clinicaltrials.gov, but appeared on the Japanese clinical trial registry and the OncologyPipeline database earlier this month. There had been hopes that belrestotug would enter phase 3 last year, with iTeos insisting that phase 2 results weren’t a gating factor, though logic dictated that GSK, which licensed belrestotug for $625m in 2021, wanted to see more data before pulling the trigger. Once full results of Galaxies Lung-201 – concerning precisely the same setting as Lung-301 – are published the chances of phase 3 success can be assessed.
1363













