
Ideaya socks it to GSK
Two years after being snubbed by GSK, Ideaya shows the promise of MAT2A inhibition.
Two years after being snubbed by GSK, Ideaya shows the promise of MAT2A inhibition.

GSK might today be looking carefully at the clinical data just released on Ideaya’s MAT2A inhibitor IDE397; the UK company once held rights to opt into this project, but decided against taking these up in August 2022, preferring to focus its synthetic lethality efforts on other mechanisms.
Two of those, Pol theta and Werner helicase inhibition, are still in play, courtesy of two other Ideaya assets to which GSK does retain rights. As such, Ideaya will be careful not to criticise GSK’s 2022 snub, but its message is clear: the new data position MAT2A as a viable new mechanism, and IDE397 as potentially its first exponent.
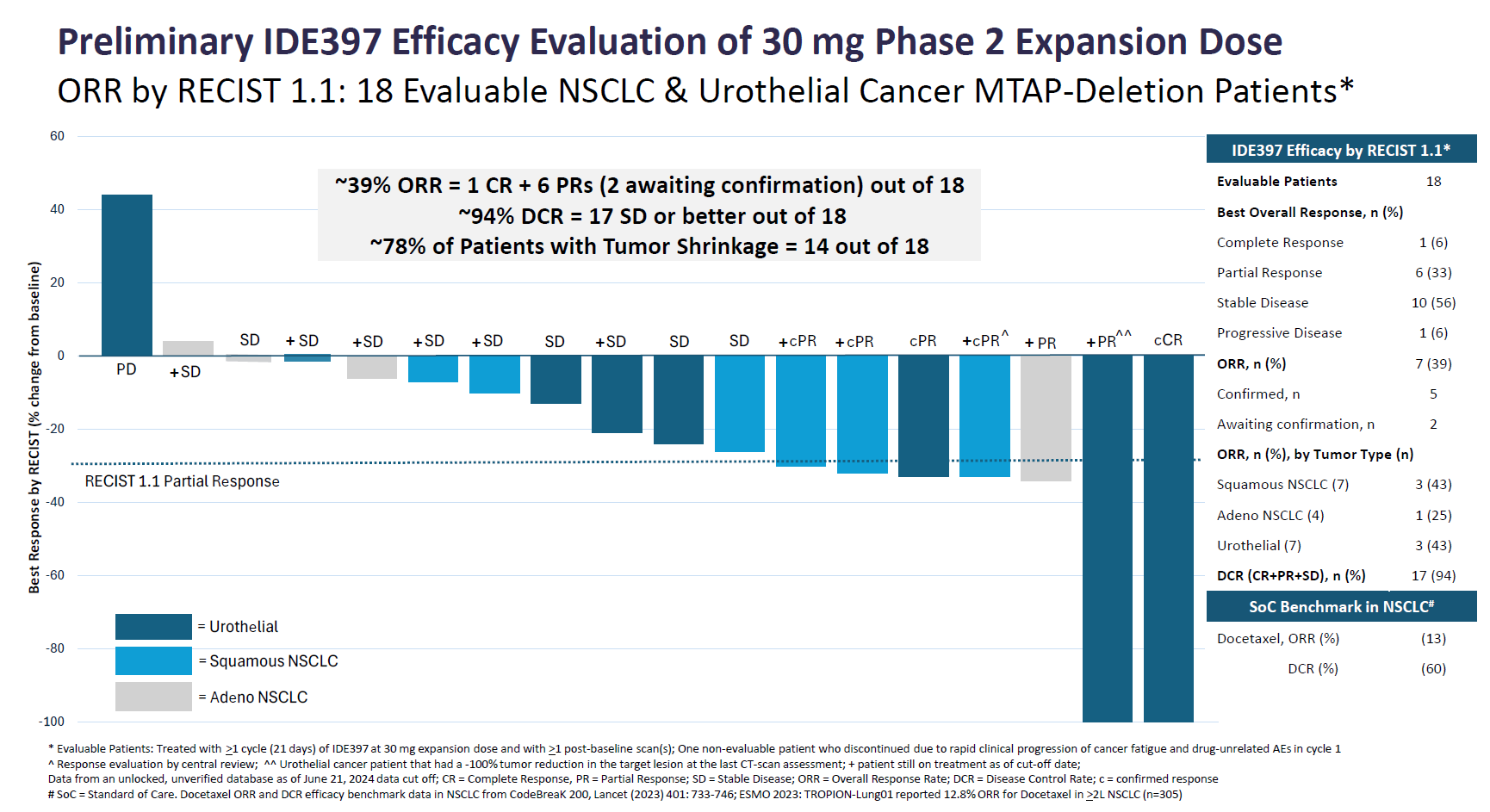
MAT2A (methionine adenosyltransferase 2A) is an enzyme whose inhibition could be used to treat solid cancers that have a mutation called MTAP deletion. Ideaya reckons MTAP-deletion rates have been reported at over 15% in NSCLC, and over 25% in urothelial bladder cancer – the two tumours in which it today reported IDE397’s clinical activity.
The phase 1 study in question seeks to enrol 180 patients with any pretreated MTAP-deleted solid tumours, but today’s update concerned 18 – seven with urothelial and 11 with NSCLC – who were given 30mg once-daily IDE397 in dose expansion. The headline number was a 39% overall response rate.
The seven responses included a confirmed complete remission, in a urothelial cancer subject, and six PRs (two of which have yet to be confirmed on second scan), comprising two urothelial patients, three subjects with squamous NSCLC, and one with NSCLC with adenocarcinoma histology.
Cutting this small dataset further yields ORRs of 43% in urothelial cancer, 43% in squamous NSCLC and 25% in adenocarcinoma NSCLC. Ideaya said no drug-related serious adverse events were seen with this IDE397 dose, and no drug-related AEs led to discontinuation. However, the group revealed that “quality control failures” precluded ctDNA molecular response analysis of several patient samples.
There is a key dimension to Ideaya’s work on IDE397: the molecule is also being combined with Gilead’s Trodelvy in bladder cancer, and separately with Amgen’s PRMT5 inhibitor AMG 193 in solid tumours.
The bull case for Ideaya, whose shares were up 15%, is that one of these clinical collaborations could become a permanent tie-up – a scenario made more likely by GSK’s abandonment, which left Ideaya with full IDE397 rights. Still, initial results for AMG 193 monotherapy underwhelmed last year, so the hope must be that the preclinical promise of an IDE397 combo can be repeated in the clinic.
Another PRMT5 player is Bristol Myers Squibb, whose MRTX1719 looks better than AMG 193.
Who else?
The IDE397 data will also be of interest to Servier and Insilico Medicine, which recently moved to start first-in-human trials of the MAT2A inhibitors S95035 and ISM3412 respectively.
Servier’s effort is especially notable. The company first got into MAT2A inhibition through AG-270/S095033, a molecule it acquired with the Agios business in 2020. However, that was discontinued after showing liver toxicity, so S95035 is a follow-on project, and its clinical development shows that Servier continues to see promise in this mechanism.
If that’s the case it’s surprising to see how little other industry activity there is in MAT2A; OncologyPipeline reveals just three other projects, all at Chinese companies and all still at the preclinical stage.
The MAT2A inhibitor pipeline
| Project | Company | Status |
|---|---|---|
| IDE397 | Ideaya Biosciences | Ph1/2 in solid tumours with MTAP gene deletions; also Trodelvy combo in urothelial, and AMG 193 combo in solid tumours |
| S95035 | Servier | Ph1 in solid tumours with MTAP gene deletions |
| ISM3412 | Insilico Medicine | Ph1 in solid tumours with MTAP gene deletions starts Mar 2025 |
| SCR-7952 | Jiangsu Simcere Pharmaceutical | Preclinical |
| SYH2039 | CSPC Pharmaceutical | Preclinical |
| BT115386 | ScinnoHub Pharmaceutical | Preclinical |
| AG-270 | Servier (ex Agios) | Discontinued in ph1/2 |
Source: OncologyPipeline.
This is an updated version of a story published earlier.
2483













