Ideaya eyes neoadjuvant uveal melanoma
But some phase 3 details are unconfirmed, and phase 2 data haven’t shed light on the biggest question.
But some phase 3 details are unconfirmed, and phase 2 data haven’t shed light on the biggest question.

Ideaya Biosciences has seen enough with its PKC inhibitor darovasertib to begin pivotal development in neoadjuvant uveal melanoma, adding to an ongoing registrational trial in metastatic disease.
However, the primary endpoints of the upcoming trial focus on vision loss and eye preservation – unusual in oncology – and Ideaya is still nailing down other details with the FDA. Added to this uncertainty was a confusing phase 2 update, which involved two studies being presented together, and covering different patient groups tested for different outcomes.
Furthermore, during a conference call to discuss the results, Ideaya admitted that it had no direct evidence that darovasertib was fulfilling its ultimate goal of reducing metastatic disease. The company’s stock closed down 4% on Monday.
Sparing vision
The latest data came from two uncontrolled phase 2 studies, one sponsored by Ideaya and the other investigator-initiated. The company trial enrolled uveal melanoma patients scheduled for enucleation (removal of the eyeball) or brachytherapy (localised radiotherapy). The investigator-sponsored trial enrolled enucleation patients only.
Patients were given neoadjuvant darovasertib for up to 12 months, at which point they could receive primary local therapy.
Ideaya believes that darovasertib can shrink tumours enough to enable the avoidance of enucleation, and reduce radiation doses for brachytherapy patients; a side effect of the latter is vision loss.
Presenting the results on Monday the company combined the two trials, giving a total of 49 evaluable patients. Among these 49% had 30% or greater tumour reduction.
Among 31 patients who’d been candidates for enucleation, 61% had their eye spared. However, data previously presented from the investigator-sponsored study at ASCO in June showed a 75% (9 of 12) eye preservation rate; the latest update appears to have been flattered by the inclusion of the previous results.
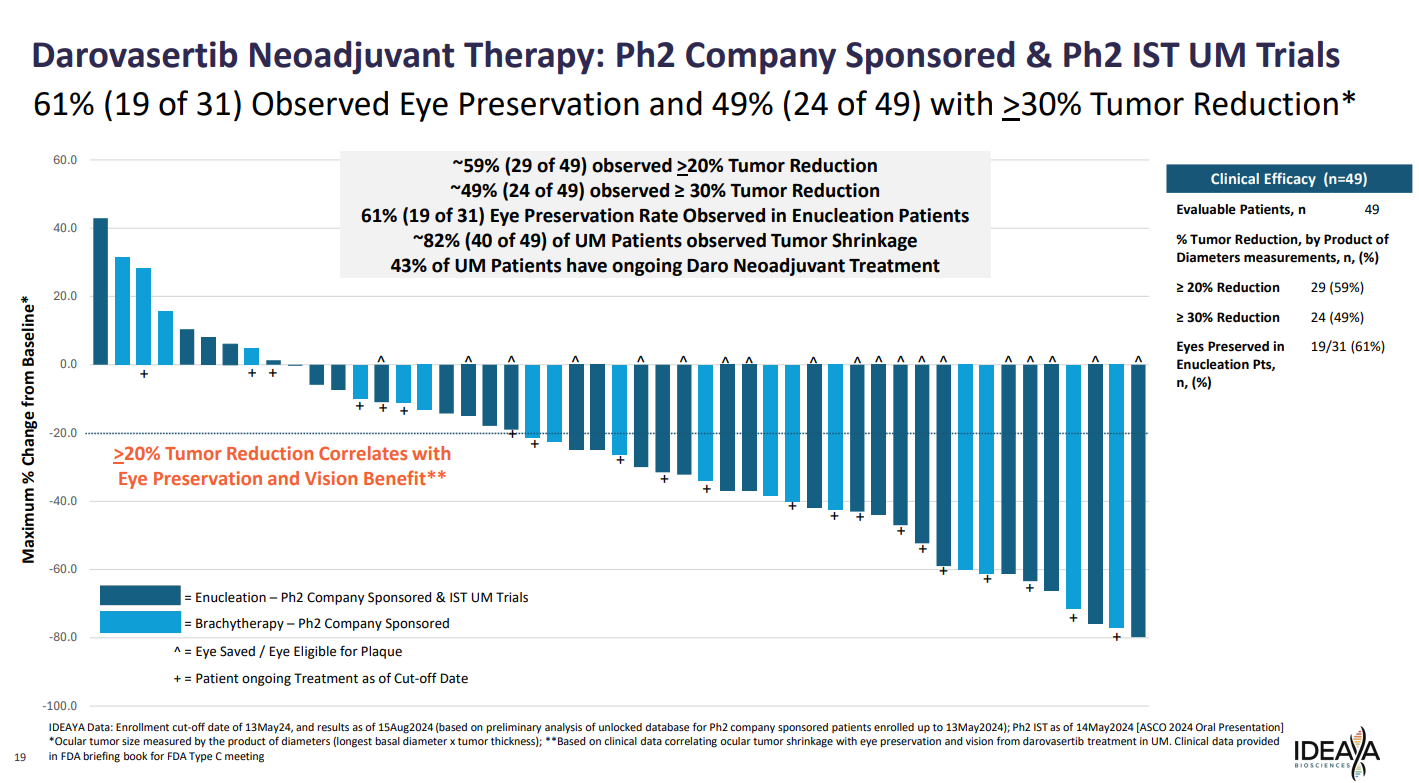
As for the brachytherapy patients, the aim here was to reduce the radiation dose. Ideaya said this was achieved, although this was measured via a simulation.
As well as preserving vision, the ultimate goal with darovasertib is to reduce metastatic disease. However, when asked whether there were any data to suggest that the project was doing this, the company said this couldn’t be proved definitively without a controlled study, although it did note that metastatic disease was closely correlated with tumour size.
Phase 3 plans
Hence to the phase 3 trial, slated to enrol around 400 patients with localised uveal melanoma at high risk for metastases, and candidates for enucleation or brachytherapy. Patients will be randomised to immediate local therapy versus six to 12 months of darovasertib followed by local therapy.
The primary endpoint for enucleation patients will be eye preservation, and the primary outcome for the brachytherapy patients will be time to vision loss. Ideaya said it still needed to define how the latter would be measured, but it could involve a 15-letter reduction in vision. The company added that these endpoints could support full FDA approval.
A secondary endpoint will be “no detriment in event-free survival”. Data could be available from an interim analysis in around two years.
Ideaya is also talking to the FDA about using ORR as a surrogate endpoint for “earlier approval scenarios”.
Ideaya said it hoped to finalise the phase 3 trial protocol within the next quarter, and that it could take another quarter to get the study going – putting the likely start late in the first quarter of 2025. With several unknowns, it’s perhaps not surprising that investors seemed skittish about darovasertib, the only PKC inhibitor in active development.
Still, there isn’t much competition in neoadjuvant uveal melanoma, which Ideaya estimates affects 12,000 patients per year across North America, Europe and Australia.
No therapies are currently FDA approved for this use. An investigator-sponsored phase 2 trial of Immunocore’s Kimmtrak in the neoadjuvant setting is not yet recruiting, while a phase 3 study is ongoing in adjuvant disease – which, Ideaya contended, is a “different kettle of fish”.
Kimmtrak is already approved for metastatic uveal melanoma, albeit only in HLA-A2*02:01-positive patients. Ideaya, meanwhile, hopes that darovasertib could be used across all metastatic patients – a goal shared by Replimune, which is taking its oncolytic virus project RP2 into phase 3 in metastatic disease.
857













