Genmab takes on AbbVie’s Elahere

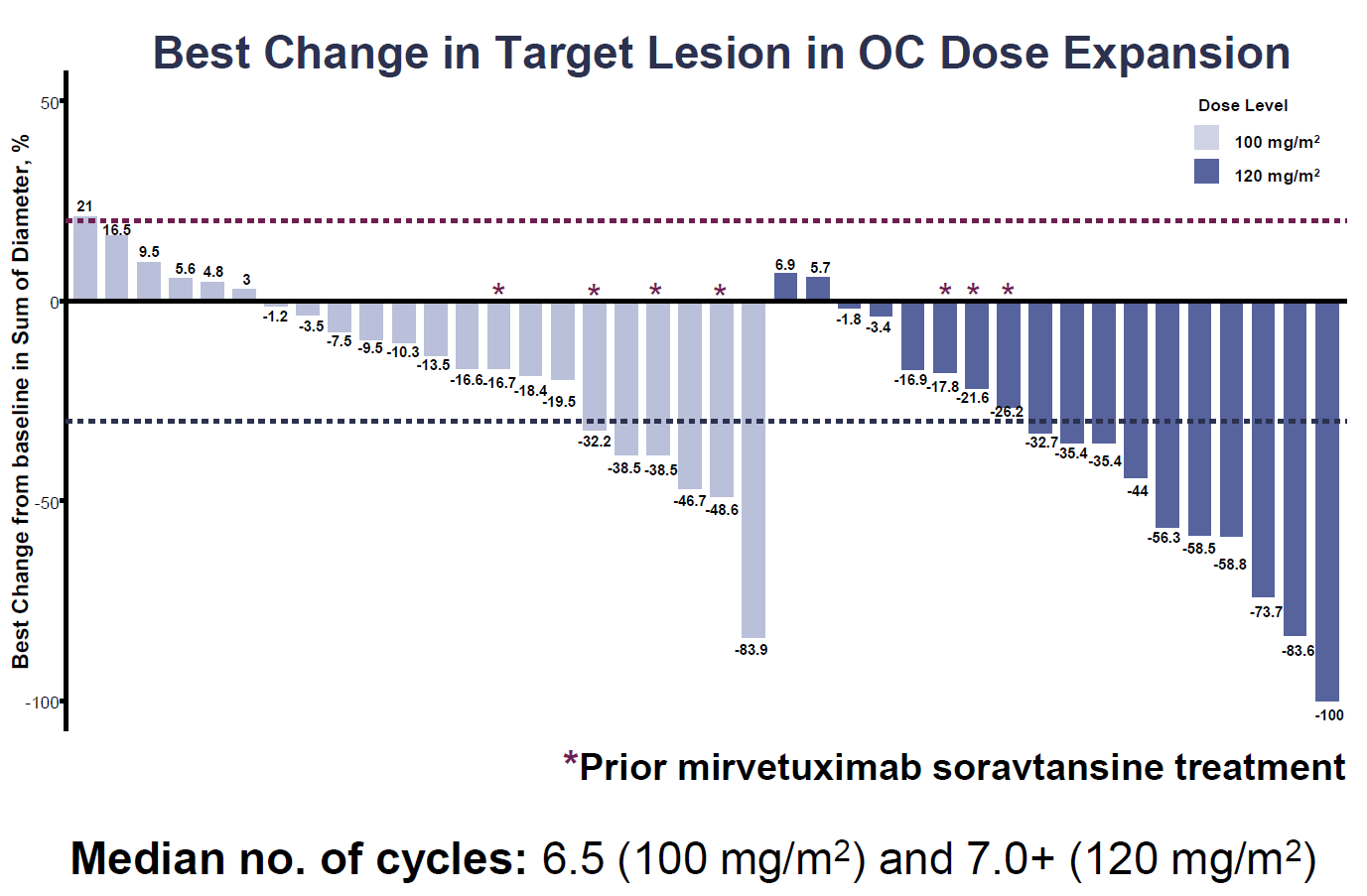
Genmab, fresh from wowing the ESMO conference with data that sent its competitor Sutro down 39%, is moving its anti-folate receptor α ADC rinatabart sesutecan into phase 3. The project lay behind Genmab’s $1.8bn takeover of ProfoundBio, and a promised pivotal trial has now begun, according to a new clinicaltrials.gov listing. This will test rina-S versus chemo in 530 patients with platinum-resistant ovarian cancer. A key enrolment criterion is the allowance of prior therapy with AbbVie’s Elahere – a clear challenge to that marketed anti-FRα ADC. Patients don’t have to express FRα unless they’ve previously had Elahere. These two features are a nod to the ESMO phase 1/2 data (the only other human trial of rina-S), in which responses were said to be irrespective of FRα status, and a handful were seen in patients pretreated with Elahere. That study, in endometrial as well as ovarian cancers, showed a 31% ORR across two doses, with the higher, 120mg/m2, yielding 50%; serious TEAEs and discontinuations were similar across the two doses, and there were no ocular toxicities, neuropathy or ILD. 120mg/m2 was deemed the go-forward dose, so presumably this is the one that’s just gone into phase 3.
Rina-S phase 1/2 data
2445













