FDA critics barely lay a glove on Geron
Imetelstat appears headed for approval after its US adcom turns into a damp squib.
Imetelstat appears headed for approval after its US adcom turns into a damp squib.

Briefing documents revealed that the FDA had serious problems with imetelstat, but in the event critics at yesterday’s advisory panel could barely lay a glove on the Geron telomerase inhibitor, which now seems headed for US approval in myelodysplastic syndromes.
On the question of whether imetelstat’s benefits outweighed its risks the vote went 12 to 2 in favour; Geron stock, which was halted during the adcom, opened this morning up 80%. A key factor driving the investment case is a takeover or licensing deal, and a haematology player like Novartis, Incyte or Bristol Myers Squibb might see the attraction of an approvable MDS drug and Geron's $1bn valuation.
Whether and what type of a deal materialises will depend on several things, not least a view on what antitrust agencies might say about overlaps with existing MDS/myelofibrosis franchises. Moreover, it’s one thing to have an approved drug and another to be able to sell it at a profit, and real-world uptake will only become apparent if and when imetelstat is launched.
The case against
At least most of the doubts about imetelstat’s pivotal Imerge trial, revealed this week in briefing documents that hit Geron’s share price, have been allayed.
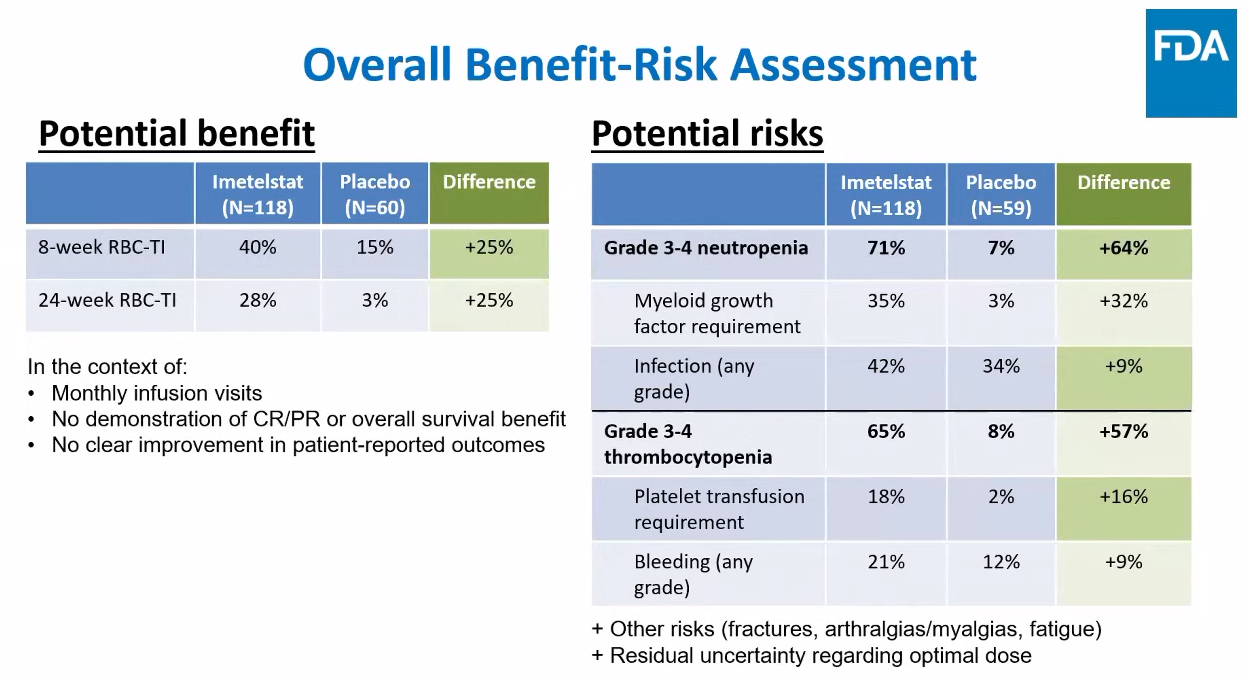
Putting the case against imetelstat the FDA’s clinical reviewer Dr Nina Kim acknowledged that Imerge met the primary endpoint of eight-week transfusion independence versus placebo, but questioned its meaningfulness. This was especially pertinent given the lack of overall survival benefit (updated analysis showed a 0.98 hazard ratio) and the fact that median duration of transfusion response was very short if all patients were considered.
This second point seemed strange since it’s standard practice when analysing response duration to look only at those patients who respond, something Geron argued. Kim insisted that with transfusion independence the measure wasn’t “binary”, involving “more of a continuum”, and since all patients had some duration recorded all should be considered.
She also slammed Geron’s patient-reported outcomes data, saying these weren’t compelling and could be due to chance, and added concerns about limited dose-finding work Geron had done before starting Imerge, something evidenced by high rates of dose modification that had to be done in the trial. She also mentioned that only 6-7% of Imerge patients had received Bristol’s recently approved Reblozyl – a fact that could become relevant to imetelstat’s real-world use.
Ultimately, few of these points gained traction with panellists, and discussion centred on imetelstat’s safety. Surprisingly, Hy’s law was barely mentioned apart from Geron’s repeated assertion that there were “no confirmed Hy’s law cases” based on lab results in Imerge, and instead the focus with adverse events fell on cytopenias in the many patients who derived no benefit.
Such arguments failed to sway 12 of the 14 panellists. The two votes against came from University of Virginia’s Dr Mark Conaway, citing the small benefit and hard to interpret responder analysis, and the NCI’s Dr Ravi Madan.
Madan, the panel chair, said Imerge data were encouraging in a significant minority of patients, but that this didn’t outweigh the fact all were exposed to risk. He said Geron needed to define the minority of responders; Dana-Farber’s Dr Toni Choueiri asked for biomarker analyses as a way of characterising responders, but ultimately tendered what he called a “narrow yes” vote.
Even the consumer representative David Mitchell, of the patient organisation P4AD, which frequently criticises the high prices of drugs that deliver little benefit, was in favour of imetelstat, saying cytopenias appeared manageable, and calling the transfusion independence endpoint of Imerge a “critical element” for patients.
Assuming that the FDA doesn’t go against the panel’s positive vote – unlikely given historical precedent, though theoretically imetelstat could be restricted to a minority of MDS patients – Geron now looks set to have its first approved drug since being founded all the way back in 1990. The PDUFA date is 16 June.
How a small biotech like Geron goes about marketing imetelstat is a separate problem, but the bull case is that a potential launch will be in someone else’s hands.
1571