
ESMO-IO 2023 – Coherus’s combo strategy takes shape

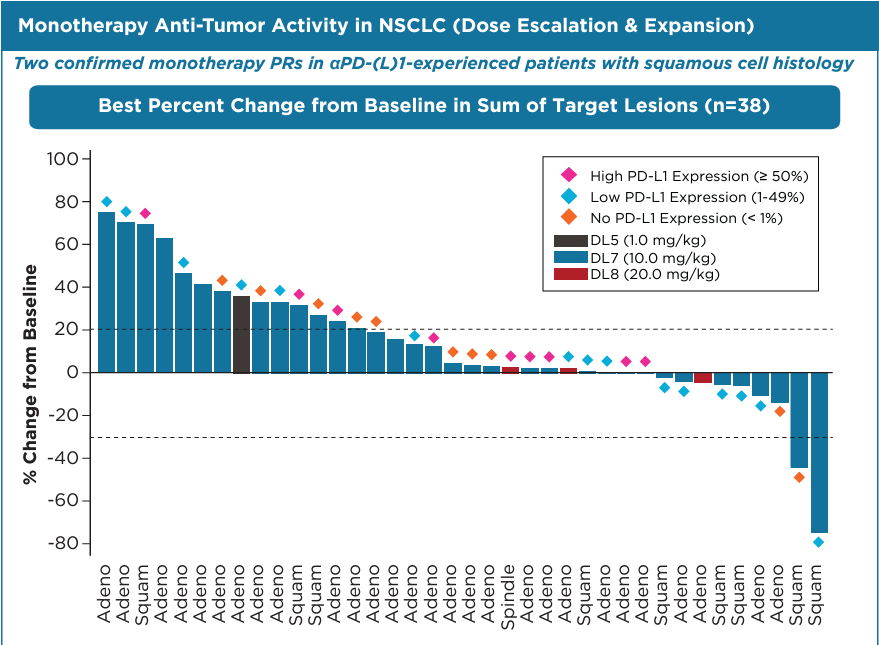
When Coherus bought the distressed biotech Surface Oncology in June, the obvious next step was combining that group’s assets with its own PD-1 blocker Loqtorzi. And that is what Coherus is now doing with the Surface-originated IL-27-targeting antibody casdozokitug, off the back of early-stage data being presented this week at the ESMO-IO meeting. At first glance the result, two partial responses among 38 late-line NSCLC patients receiving casdozokitug monotherapy, looks unimpressive. But both responders had squamous disease, low or no PD-L1 expression and had previously received PD-(L)1 inhibitors – a tough-to-treat population. The phase 1/2 trial also tested casdozokitug plus Keytruda, but here there were just six patients’ worth of data, with limited follow-up, and a best response of stable disease. Coherus has shifted to a pairing of Loqtorzi plus casdozokitug, the only IL-27 blocker in clinical development. A new cohort of the trial will test this combo in checkpoint inhibitor-refractory patients, but first-line disease could be the ultimate target. The FDA greenlit Loqtorzi recently for the niche use of nasopharyngeal carcinoma, giving Coherus an approved backbone for combos. The company has priced Loqtorzi at $8,892 per vial, 20% cheaper than Keytruda’s list price.
Efficacy data with casdozokitug presented at ESMO-IO
1095













