
ESMO 2024 – Incyte tries again in anal cancer
Having failed to get Zynyz approved in the second-line setting the group has the front line in its sights.
Having failed to get Zynyz approved in the second-line setting the group has the front line in its sights.

Quietly, almost in stealth mode, Incyte continues to progress its anti-PD-1 drug Zynyz. ESMO on Saturday saw the unveiling of late-breaking results at its presidential session from a pivotal trial in squamous cell carcinoma of the anal canal (SCAC), which the company hopes will become the drug’s second approved indication.
That’s at the second time of asking; Zynyz was initially filed for second-line SCAC, but the FDA refused to grant accelerated approval, and the new dataset, from the controlled Pod1um-303 study, concerns a front-line setting. The group is now shooting for a year-end filing, but one feature of the ESMO dataset, a so far unconvincing overall survival result, has yet to mature.
Pod1um-303, which compared Zynyz plus chemo against chemo alone, set progression-free survival as its sole primary endpoint, and on this measure it succeeded. A 37% reduction in risk of progression or death hit a p value of 0.0006, and median PFS of 9.3 months beat control by 1.9 months, the just late-breaking data revealed.
That’s enough for the authors to declare the Zynyz/chemo combo a “new standard of care”, but overall survival – a vital though secondary endpoint in Pod1um-303 – has so far shown only numerical backing. 30% reduction in risk of death looks impressive on the face of it, but the 95% confidence interval’s upper bound exceeded 1.00, and the presenting author, Royal Marsden Hospital's Dr Sheela Rao, said there was only a trend to significance.
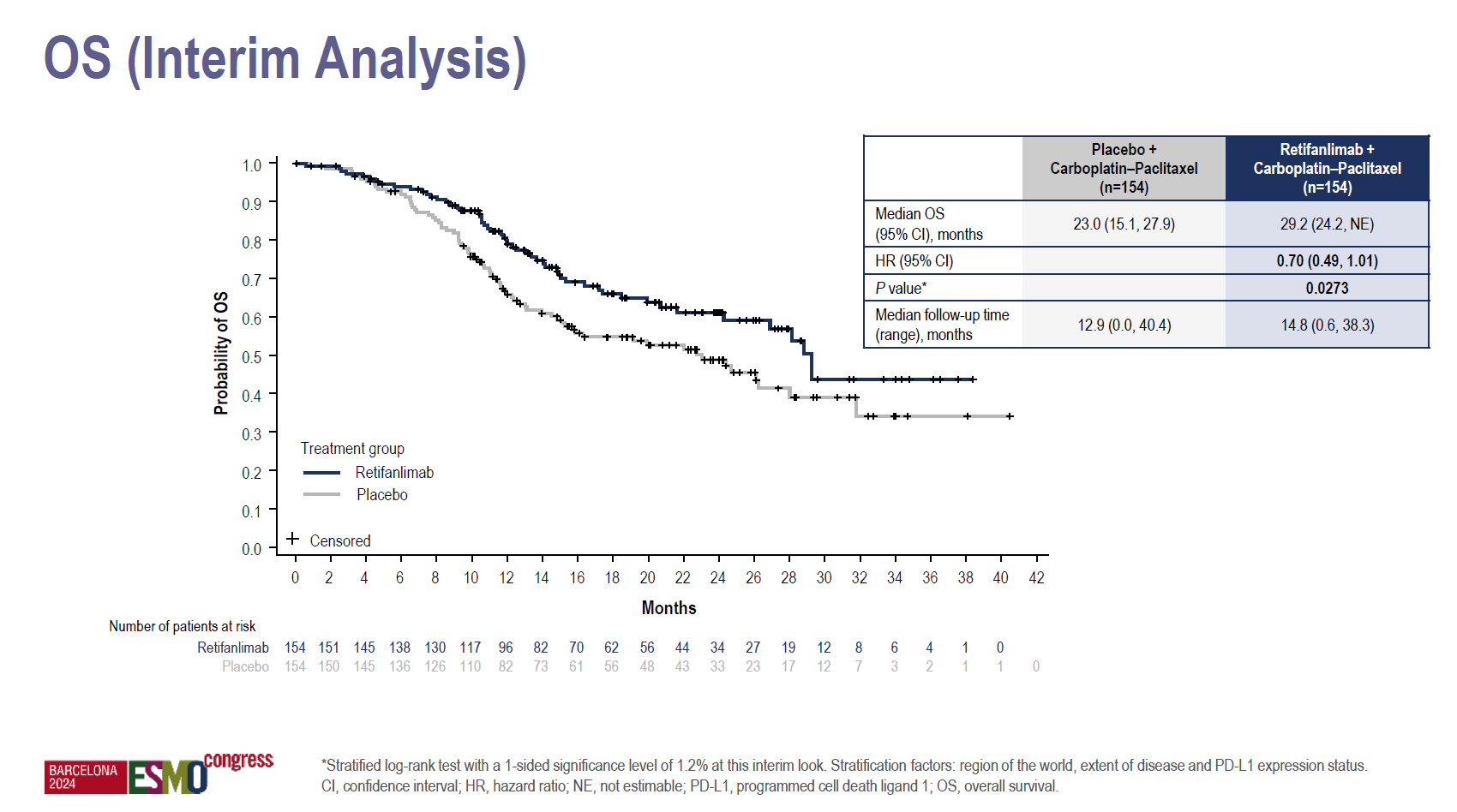
Still, interim OS curves showed a clear separation, and ultimately, even if mature OS data fail to hit significance, such a result might not matter much. SCAC is a relatively niche indication, without advanced treatment options, where chemo remains a standard of care.
Lilly’s Erbitux, approved for colorectal cancer, is sometimes used in SCAC, and a small, investigator-sponsored trial tested its chemo combo in the first-line setting. Here three-year survival was 89%, versus 79% for chemo alone, and an ORR of around 64% in both cohorts appeared meaningfully higher than in Pod1um-303, at least on this cross-trial comparison.
Another investigator-initiated effort was the phase 2 Vital study of Amgen’s Vectibix, a molecule with a similar mechanism of action to Erbitux, also in first-line SCAC. However, here ASCO 2018 reported three-year rates of OS and PFS of 78% and 57% respectively, which the authors said came up short of expectations.
Incyte’s first attempt at getting Zynyz approved, for chemo-refractory SCAC, was hit by a CRL in 2021 after an adcom voted 13-4 that the uncontrolled Pod1um-202 study was insufficient to back accelerated approval, and that a decision should be deferred pending data from Pod1um-303.
In the meantime, Zynyz was approved in another setting, second-line Merkel cell carcinoma, but the submission was so low key that Incyte didn’t even announce its filing. Even if Pod1um-303 does lead to another approval Zynyz, the eighth anti-PD-(L)1 MAb to reach the US market, seems destined to play only a supporting role, perhaps as the backbone for other Incyte immunotherapy combos.
Efficacy summary from Pod1um-303
Zynyz + chemo | Chemo | |
|---|---|---|
| PFS | 9.3mth | 7.4mth |
| HR=0.63 (0.47, 0.84), p=0.0006 | ||
| OS | 29.2mth | 23.0mth |
| HR=0.70 (0.49, 1.01), p=0.0273 | ||
| ORR | 55.8% | 44.2% |
Source: ESMO.
This is an updated version of a story published earlier.
700













