
ESMO 2024 – changing FDA stance could haunt Astra’s Niagara win
Niagara looks sound, but how much benefit did Imfinzi contribute to each of its two settings?
Niagara looks sound, but how much benefit did Imfinzi contribute to each of its two settings?

AstraZeneca could be heading for a showdown with the FDA over the potential approval of Imfinzi for perioperative treatment of bladder cancer, based on the Niagara study. The problem isn’t Niagara’s result – an ESMO late-breaking presentation on Sunday revealed this to be highly positive – but rather its design.
Niagara gave muscle-invasive bladder cancer patients Imfinzi plus chemo in the neoadjuvant stage, followed by radical cystectomy and then Imfinzi monotherapy as adjuvant. With data only available backing Imfinzi’s effect over the entire perioperative course it’s impossible to discern how much of the drug’s benefit was down to it being given pre and how much post-operatively.
That’s a live issue because of a US advisory committee meeting convened in July precisely to discuss the robustness of trials with this perioperative design. Primarily the adcom concerned another Astra trial, Aegean in perioperative NSCLC, but its outcome was clear: these studies should use a more complex design to spell out individual contributions of treatment stages.
Niagara first
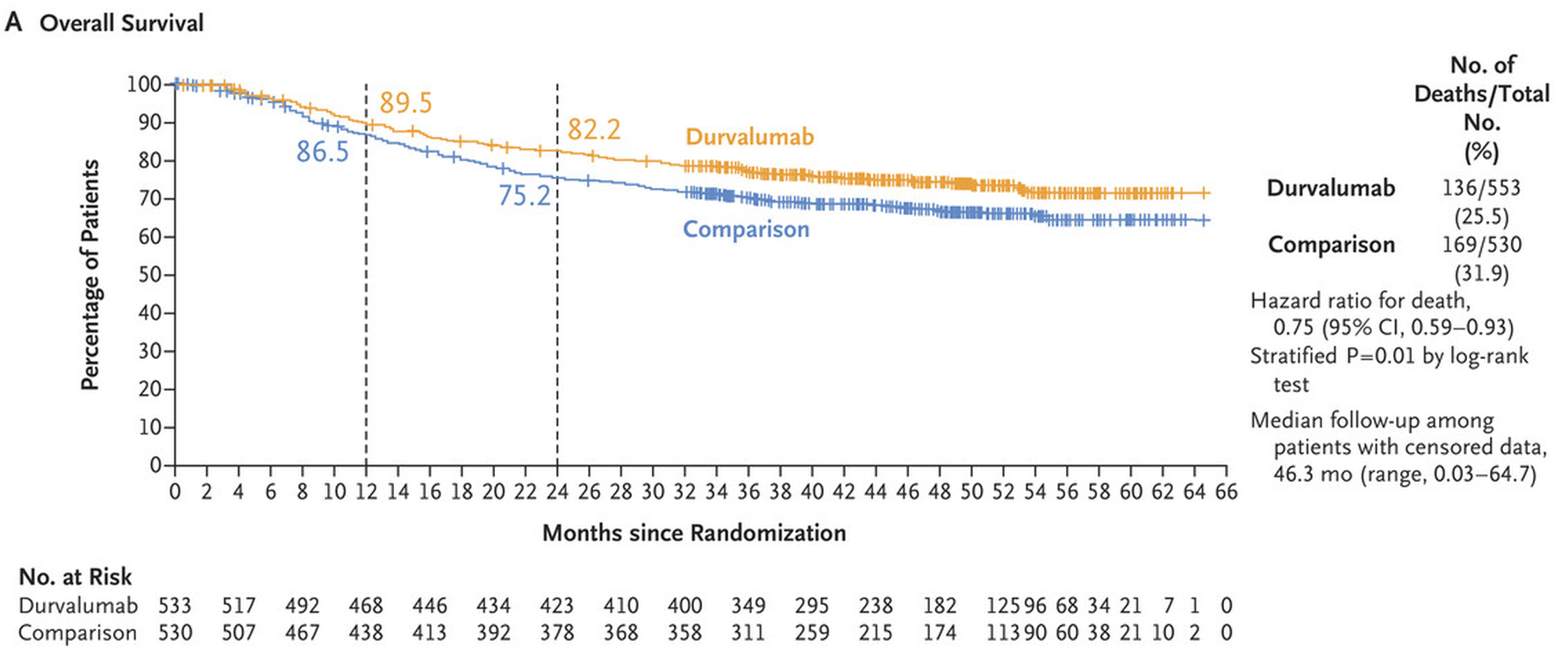
Astra already toplined Niagara in June, saying the trial was statistically significant and clinically meaningful both for the co-primary endpoint of event-free survival, and the much more robust overall survival metric, set as a key secondary.
This made Niagara the first trial of an immunotherapy given before and after surgery to extend survival in bladder cancer. The ESMO presentation bore this out: at a median 42.3 months’ follow-up the hazard ratio for EFS came in at 0.68 (p<0.0001), while on OS the perioperative Imfinzi course cut risk of death by 25%, with a landmark analysis showing 82% of Imfinzi patients alive at 24 months, versus 75%.
One cross-trial comparator for Niagara is Bristol Myers Squibb’s Checkmate-274, where Opdivo delivered a 19.1-month median OS benefit, and cut risk of death by 24%, versus placebo. However, this was a study specifically in the adjuvant setting, and backed Opdivo’s approval in this bladder cancer use alone.
Niagara had a second co-primary, pathological complete response, but on this measure Imfinzi failed, ESMO revealed; however, relative to EFS and especially OS, pCR is a less important, subjective measure, so this failure might not matter much. The comparator for all these endpoints was neoadjuvant chemo, followed by surgery alone.
Perioperative elephant
However good the Niagara data are, the elephant in the room is the inability to discern how much of Imfinzi’s benefit accrues in which setting, and whether it’s necessary to expose patients to a course of this drug, with concomitant side effects, for the entire perioperative period.
In a press conference ahead of Sunday's presentation of full Niagara data at ESMO’s presidential session, the National Cancer Center Singapore’s Professor Rebecca Dent, the meeting’s scientific chair, agreed that whether it was necessary to continue active treatment in the adjuvant stage in a trial with this design had emerged as a “difficult but important question to ask”.
“That’s a bit of a mysterious question, and I suspect it’s individualised for some patients who do [need to continue on Imfinzi] and some patients who don’t,” she told journalists. “Are the current generation of trials set up to answer that question? Probably not.”
Assuming that Astra files the Niagara data with the FDA it will then be up to the US agency to decide how strictly to follow the July adcom’s recommendation. That vote was unequivocal, by 11 to none, that to back perioperative approval trials should split out a drug’s separate benefits, though for Aegean an exception was made – this trial did result in Imfinzi’s approval last month – on the grounds that Aegean was set on its current path six years ago.
Whether the FDA makes a similar exception for Niagara is now a key consideration.
This is an updated version of a story published earlier.
2698













