
ESMO 2024 – Bristol abandonment overshadows Immatics

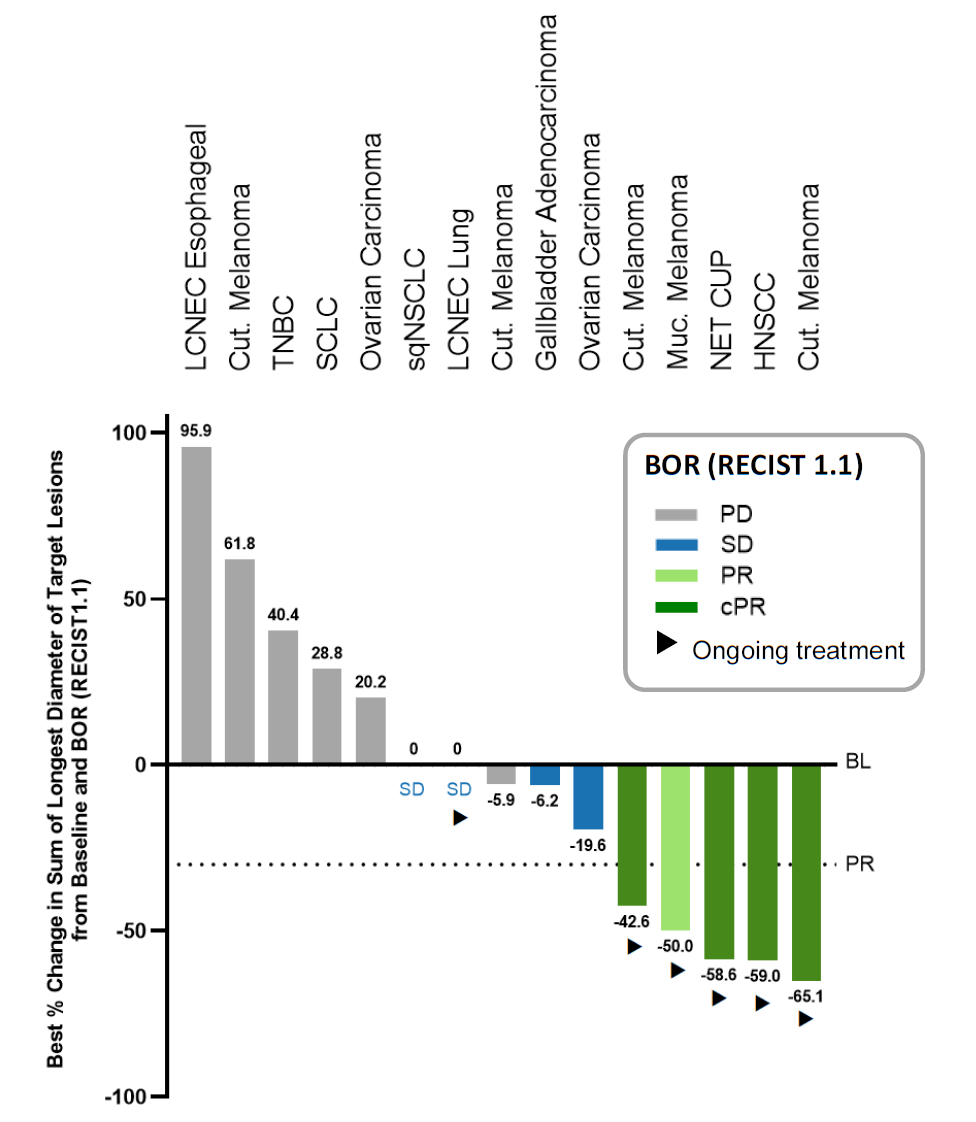
After Immatics’ success against the PRAME antigen the company came to ESMO with first human data backing a second T-cell receptor project, coded IMA401 and targeting MAGE-A4/8. Unfortunately the results weren’t convincing enough for the biotech’s partner, Bristol Myers Squibb, which has terminated the collaboration. The deal was signed nearly three years ago, and gave Immatics $150m up front, so at least that’s cash in the bank. As for the IMA401 data, across an efficacy-evaluable population of 29 patients Immatics reported a 21% ORR, including unconfirmed responses, seemingly decent for a cohort of solid tumour subjects. Drilling further down into just the 17 patients who had high expression of MAGE-A4/8, and who got the highest two IMA401 doses, showed a 29% ORR, with responses in melanoma – not especially surprising – and, more impressively, neuroendocrine tumour of unknown primary and head and neck cancer. Treatment-related adverse events were seen in 80% of patients, and at grade 3 or above in 54%, but Immatics said a maximum tolerated IMA401 dose hadn’t been reached. If this is enough to back continued development of IMA401 this will be work Immatics will now be funding on its own.
IMA401 activity at dose levels 6-7, in MAGE-A4/8-high expressers
1322













