ESMO 2023 – regulators come under fire for Retevmo trial requirements

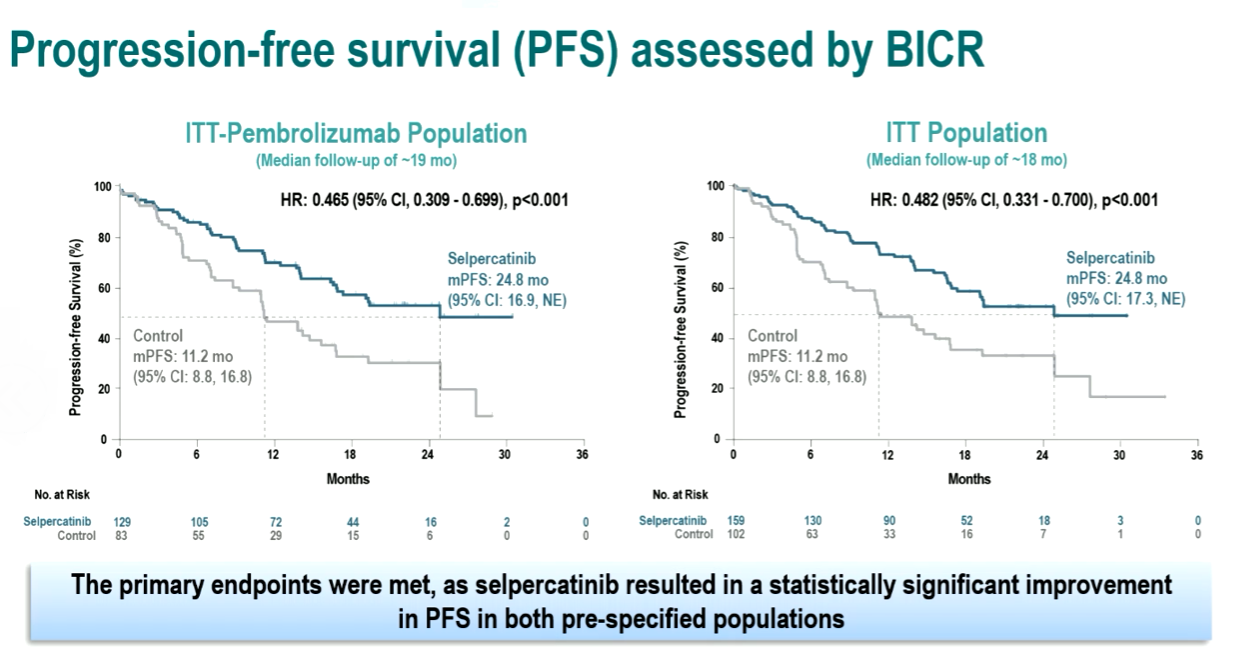
Lilly’s RET inhibitor Retevmo, gained through its $8bn purchase of Loxo in 2019, was confirmed as the first-line standard of care in RET-fusion-positive NSCLC at ESMO on Saturday. But the discussant, Gustave Roussy’s Professor Benjamin Besse, questioned the need for the randomised, controlled Libretto-431 trial in the first place. Retevmo already has full approval in the US, based on the uncontrolled Libretto-001 study, but the EMA asked for randomised data as part of its 2021 conditional approval, Besse noted, which has had a knock-on effect on payers. Besse cited the rare incidence of RET rearrangements – which occur in just 1.7% of NSCLC patients – and a lack of options for these patients. Indeed, he questioned whether it was ethical to give control patients chemo, given the lack of evidence of efficacy in RET-fusion patients. In the event, Libretto-431, which was published simultaneously in the NEJM, found an 84% overall response rate with Retevmo – the same result seen in Libretto-001. In Libretto-431 progression-free survival, the primary endpoint, was almost doubled in the Retevmo arm versus standard of care, at 24.8 months versus 11.2 months respectively.
1171













