
ESMO 2023 – red flags for PSMAfore
The study of Novartis’s Pluvicto in pre-chemo prostate cancer is a bust on overall survival, with questions raised about trial design.
The study of Novartis’s Pluvicto in pre-chemo prostate cancer is a bust on overall survival, with questions raised about trial design.

Today a discussant at ESMO poured cold water on the idea that Novartis’s radiopharmaceutical Pluvicto should be the therapy of choice after androgen receptor-directed therapy but before chemo in castration-resistant metastatic prostate cancer.
It was revealed today that the PSMAfore study in this setting didn't show an overall survival benefit with Pluvicto versus androgen receptor-directed therapy at an interim analysis. The discussant, the University of Adelaide’s Dr Christopher Sweeney, concluded that Pluvicto did “not replace” any existing options in the disease, but was merely “one chess piece on the chessboard”.
A key part of his criticism involved questions about Novartis’s trial design. Patients in PSMAfore had progressed on one second-generation androgen receptor pathway inhibitor, such as Zytiga or Xtandi, and were randomised to receive either Pluvicto or a different androgen-receptor-targeting therapy.
But hormone-switch therapy was a “weak control”, Sweeney told ESMO attendees today. A patient is unlikely to respond to one anti-androgen therapy when they've already failed on a similarly acting drug, and chemo has been moving into earlier stages of the treatment paradigm.
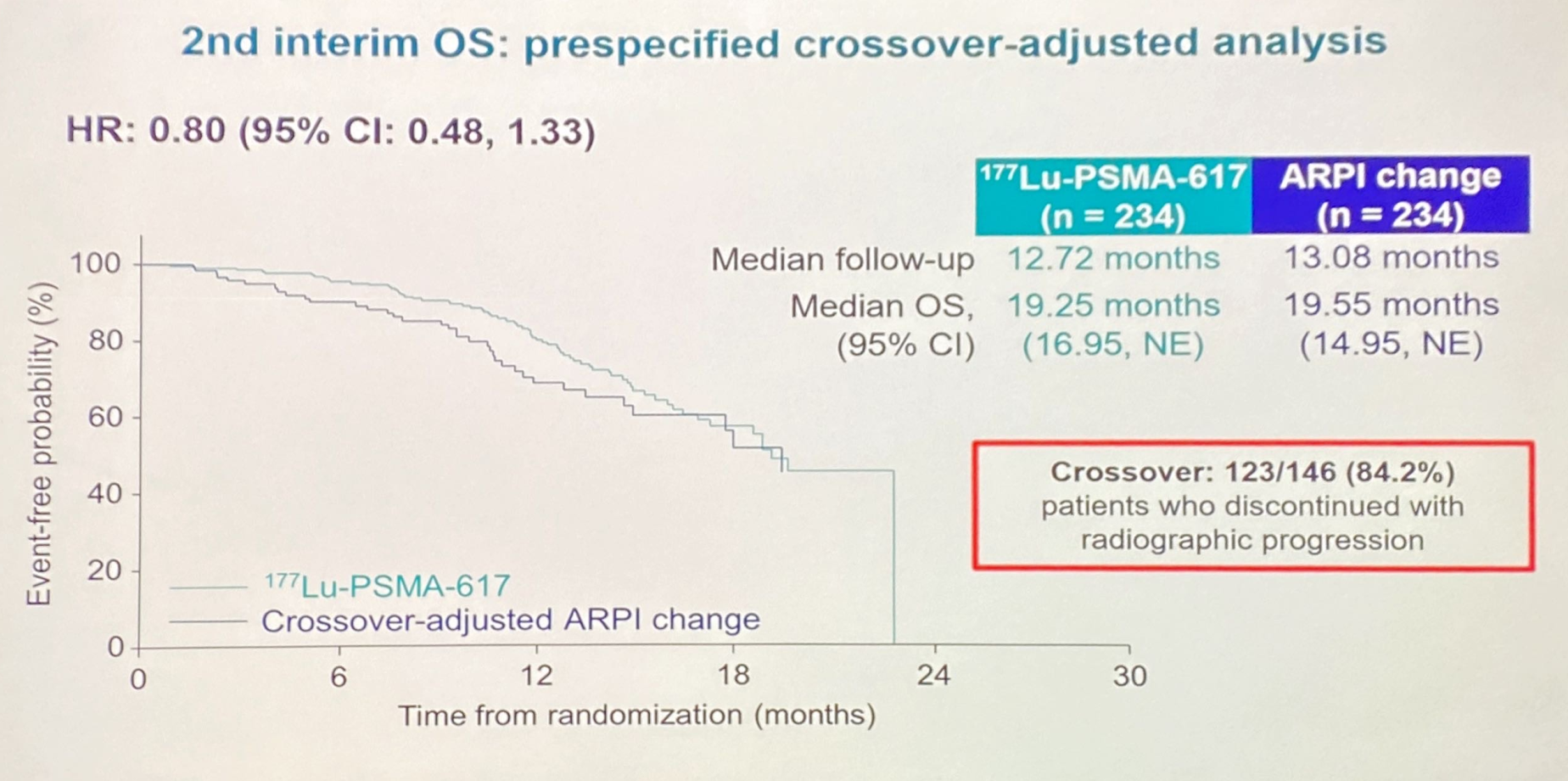
To make up for this unsatisfactory control arm, Novartis allowed patients to cross over to Pluvicto. 84% of patients – possibly a record for a phase 3 study – did so, obliterating any chance of the trial showing an OS benefit. And, even adjusting for this crossover, it’s hard to see anything but hints of a numerical OS advantage.
It shouldn't be forgotten that PSMAfore hit its primary endpoint, progression-free survival, as revealed in an ESMO abstract last week, with Pluvicto-treated patients showing PFS of 12.0 months, versus 5.6 months for control.
Sweeney acknowledged that Pluvicto, which is already approved post-chemo, is an effective therapy. But PSMAfore has failed to answer the key question: in which order should prostate cancer therapies be given?
If the question is whether the correct sequence is Pluvicto then chemo, rather than chemo then Pluvicto (the Novartis drug's approved use), then PSMAfore doesn't answer it. Neither was the trial designed to do so.
The latest findings don't bode well for the Splash trial of Point Biopharma’s rival PSMA-targeting radiopharmaceutical PNT2002, which has a similar design. Lilly paid $1.4bn for Point earlier this month.
Pluvicto might well get approved in the pre-chemo setting. Novartis said during an investor event that a 2023 filing was off the table, and it would instead look to submit Pluvicto next year, using data from the next OS analysis.
This story has been updated
3097













