
ESMO 2023 – Padcev hits its home run
The presidential session heralds a combo of Padcev and Keytruda as the new front-line bladder cancer standard of care.
The presidential session heralds a combo of Padcev and Keytruda as the new front-line bladder cancer standard of care.

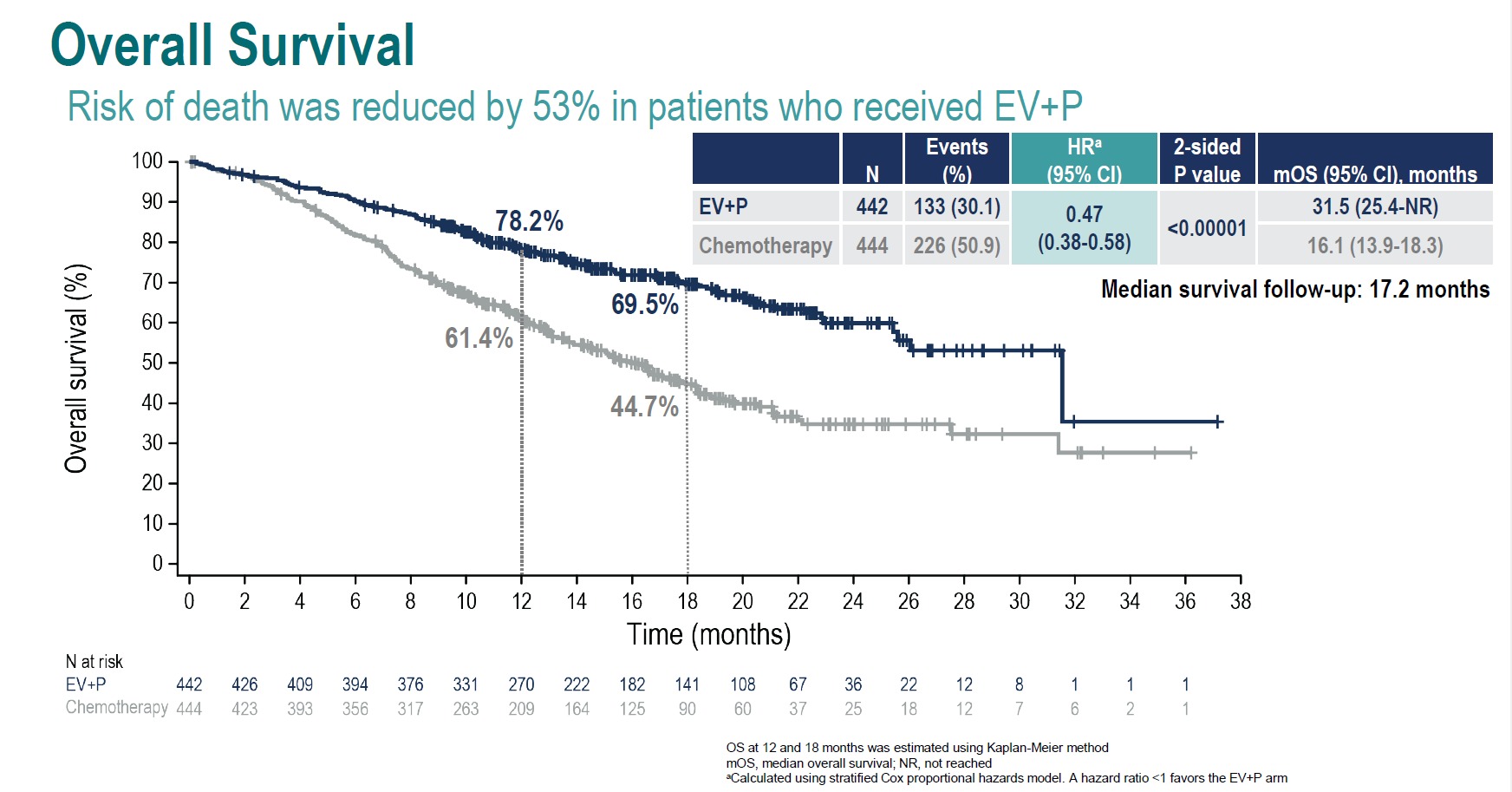
The EV-302 study was meant to confirm the accelerated approval of Astellas/Seagen’s Padcev plus Keytruda in front-line urothelial bladder cancer, but in the event it’s defined the new standard of care for this disease.
The data, just presented at ESMO’s presidential session, mark the first time platinum chemo has been beaten on overall survival in the fist-line setting. Not only will the results allow Pfizer, soon to become Padcev’s owner, to breathe a sigh of relief, they could make life difficult for Bristol Myers Squibb, Merck KGaA and Bicycle Therapeutics.
It’s fair to say that the Padcev/Keytruda combo blew EV-302’s chemo comparator out of the water, with a hazard ratio indicating that risk of death was cut by 53% across the study. The result was irrespective of either patients’ PD-L1 status or their cisplatin eligibility, said Professor Thomas Powles, of Barts Cancer Institute, adding that cisplatin eligibility now became less relevant.
The outcome should make Padcev’s formal US approval across three bladder cancer settings, including the first-line Keytruda combo use of EV-302, a mere formality. It’s as important for Padcev as it is for Merck & Co’s Keytruda, whose status as a first-line bladder cancer therapy was on slightly shaky ground.
Metastatic urothelial bladder cancer has been a shifting field for immunotherapy, with Roche’s Tecentriq first restricted and ultimately withdrawn from the market. Keytruda too has been restricted, and currently carries a first-line label only in patients ineligible for platinum chemotherapy.
Meanwhile, Bristol’s Opdivo looked like becoming a contender via its chemo combo Checkmate-901 study. However, unfortunately for Bristol these late-breaking data were presented straight after EV-302, and were thus completely overshadowed: while Checkmate-901 marked another OS win over chemo, the Opdivo combo’s 21.7 months of median OS, and 0.78 hazard ratio, paled into insignificance against the EV-302 data.
Simply the best
Discussing the two abstracts, the NCI’s Dr Andrea Apolo didn’t beat about the bush: OS for Padcev plus Keytruda “is better than” Opdivo plus chemo, she told ESMO, adding that Padcev plus Keytruda now “takes first place as the best first-line regimen in urothelial carcinoma”.
She did express caution about Padcev’s side effects, which included peripheral neuropathy that can be treatment-limiting, skin reactions, eye toxicity and hyperglycaemia, saying there was a need now to reduce toxicity; and that included financial toxicity, she pointedly added.
Another question she raised is how treatment should now be sequenced. Current second-line therapy depends on whether patients have received chemo in the front line, but nothing is known about the best option for those who’ve had Padcev plus Keytruda.
It’s also worth considering the fate of Merck KGaA’s anti-PD-L1 drug Bavencio, for which first-line bladder cancer maintenance represented a beacon of hope. It might be supposed that EV-302 wipes out Bavencio’s role here, as Bavencio is given when patients are in response to chemotherapy, which might now not remain a first-line treatment for long.
Finally, spare a thought for Bicycle Therapeutics, whose lead asset, zelenectide pevedotin, is like Padcev an ADC against Nectin-4. As recently as last month, before EV-302 was toplined, Bicycle was touting FDA “alignment” on an accelerated approval path for zelenectide on the basis of a first-line Keytruda combo study that uses chemo as a comparator.
Given the outcome of EV-302 it can safely be assumed that such plans are in tatters.
3641













