
ESMO 2023 – Biontech’s Neon buy fails to shine

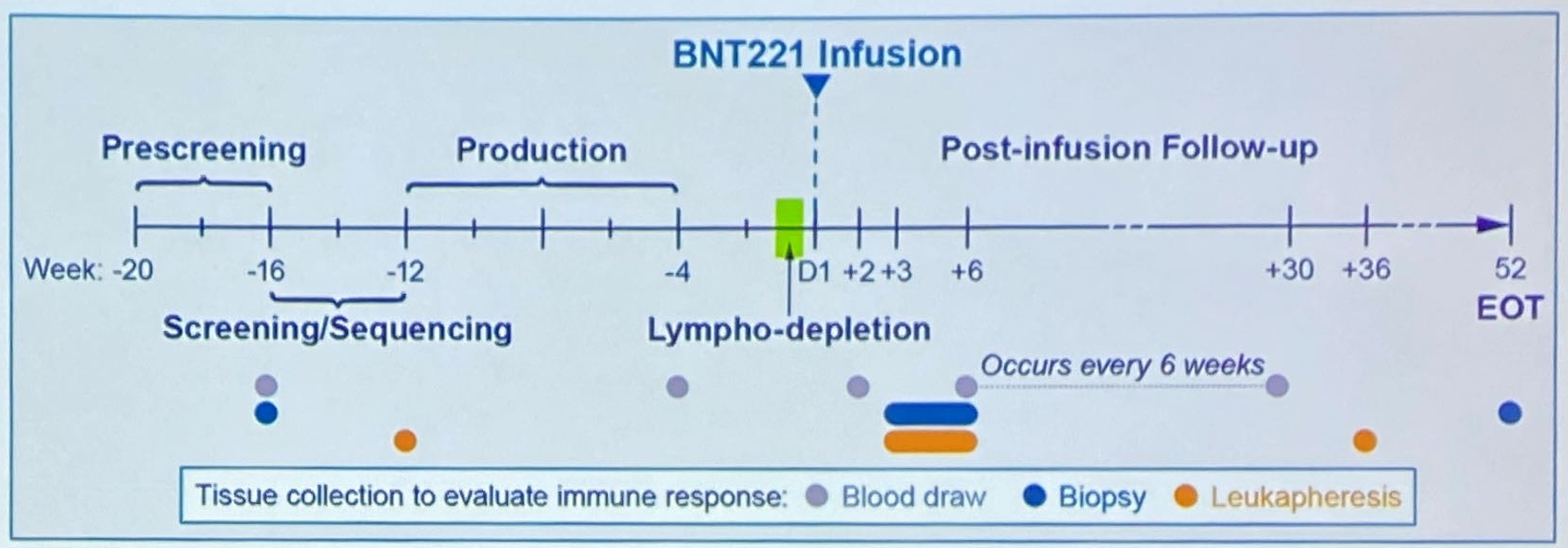
BioNTech is trying a souped-up tumour-infiltrating lymphocyte (TIL) approach with its Neon-originated project BN-221, but early results presented at ESMO today disappointed. Of nine evaluable melanoma patients who received the treatment in a phase 1 trial, there were no responses, although there was some evidence of tumour shrinkage. The discussant, Professor Inge-Marie Svane of the University of Copenhagen, saw “early signs of efficacy” in a refractory population – all patients had already received PD-1 and CTLA4 inhibitors. But the way forward for BN-221 might be limited to a combination with anti-PD-1s, a pairing that is currently being tested. Another disadvantage is BN-221’s long production time of around four months: a patient’s tumour is sampled, then their T cells are extracted before being “primed and activated” against neoantigens found in their tumour cells. The discussant suggested that regular TIL therapy was just as effective, and also highlighted the progress with mRNA-based neoantigen therapeutics, a simpler approach; here, Moderna’s mRNA-4157 has already impressed in melanoma. BioNTech is working on optimising the production process for BN-221, but its purchase of Neon looks like a damp squib. Luckily the group only paid the equivalent of $67m in an all-cash deal.
The BNT221 procedure
1715













