
EHA 2024 – In8bio bets on a registrational plan
The group's enthusiasm is backed by an academic trial of INB-100 in 10 AML patients.
The group's enthusiasm is backed by an academic trial of INB-100 in 10 AML patients.

In the battle of gamma-delta T-cell therapies In8bio is closing the gap on Adicet, though that’s largely down to investors losing faith in the latter’s disappointing CD20-targeting Car-T project ADI-001.
For its part In8bio has for the past two years touted results from an academic-sponsored study of INB-100, an allogeneic gamma-delta T-cell infusion that doesn’t involve Car modification. Yesterday brought another iteration of this dataset, at the European Hematology Association meeting, on the back of which In8bio plans to launch its own registrational trial of INB-100.
This will be a tall order considering that In8bio is still valued at under $100m. Perhaps fearing an imminent fund-raising to raise the necessary cash for the INB-100 study, to recruit 196 AML/MDS patients undergoing haploidentical transplant, investors this morning sent In8bio stock down 20%.
EHA poster
In8bio’s enthusiasm stems from a phase 1 AML study being run by University of Kansas Medical Center, which back in mid-2022 reported its first three patients going into morphological complete remission and staying alive at over a year.
At last year’s ASH conference this was updated to 10 patients, all of whom showed remissions. Six patients were evaluable at over a year at a 3 November data cutoff, and all six CRs were ongoing at this point. In8bio’s shares rose 27% on publication of the ASH abstract, and a further 29% over the weekend of the conference.
Yesterday brought a further update, in a poster at EHA concerning the same 10 patients but with a 31 May cutoff. All 10 are now said to have hit one year of ongoing CR, but there’s the first indication of response durability: two patients have had disease progression, one at 12.5 and the other at 14.7 months, while a third died of idiopathic pulmonary fibrosis while in CR at 15.5 months.
Given that this is high-risk AML the result seems impressive, though without a control it’s difficult to attribute the benefit specifically to INB-100, especially as the allogeneic gamma-delta T cells were given after patients received a hematopoietic stem cell transplant. In8bio claims that half of transplanted AML patients relapse within one year.
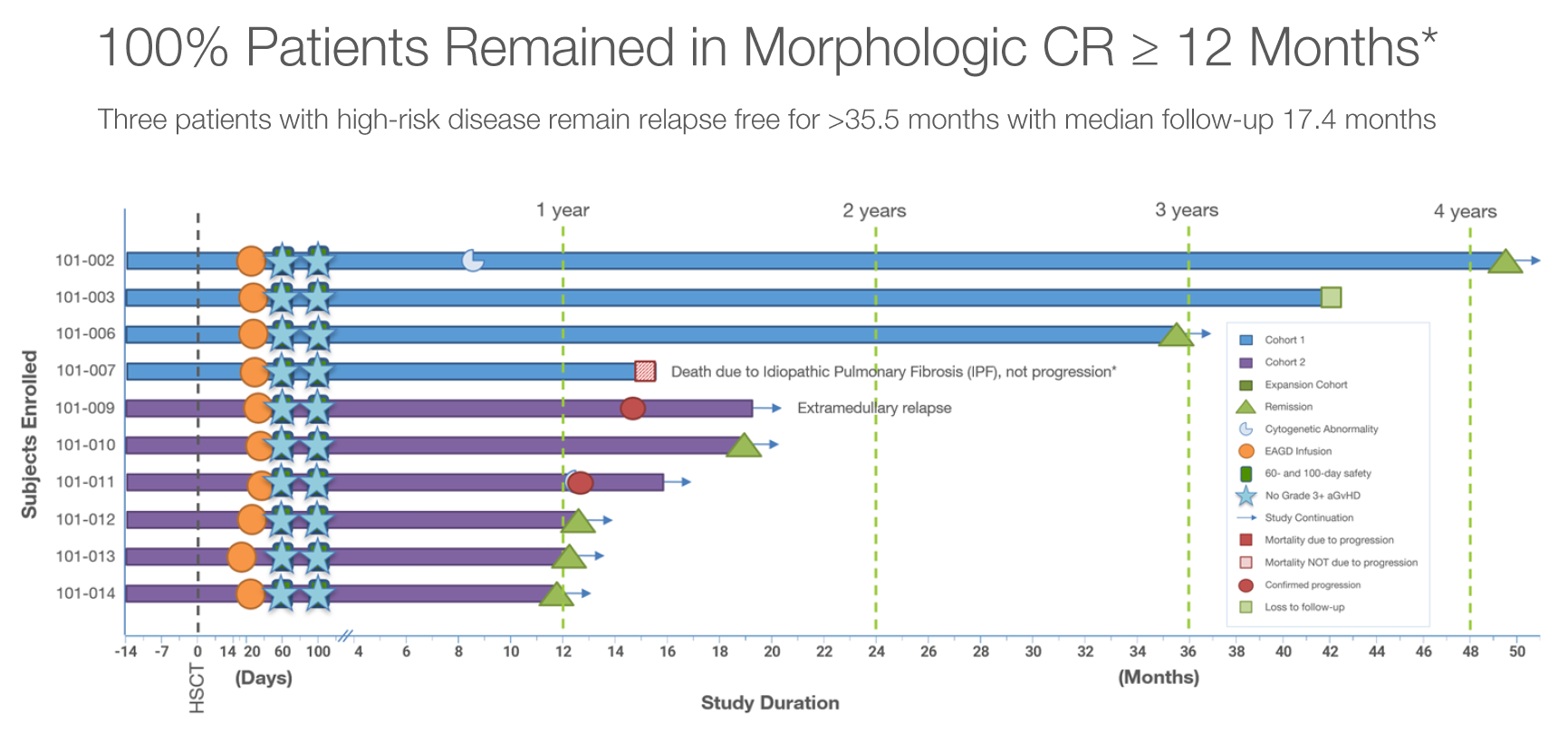
The EHA poster also revealed that 14 patients had been enrolled into this study, but one received an “out-of-specification” product and another couldn’t be dosed owing to failure to manufacture INB-100, which is derived from partially mismatched related donors. Two others relapsed or died before being dosed.
Such caveats notwithstanding, In8bio now proposes to run its own trial in a similar transplant maintenance setting in 196 AML/MDS patients. Half will be randomised to INB-100 and half to standard of care, with relapse-free survival as primary endpoint. However, this plan has yet to be discussed with the FDA.
When In8bio first reported INB-100 data in 2022 Adicet was valued at $700m after becoming the first company to show clinical promise with a gamma-delta Car-T therapy. Meanwhile, In8bio had to change its name from Incysus, took two attempts at an IPO before getting a float away, and then saw its valuation collapse.
Since then Adicet has crashed as investor hopes evaporated. Now In8bio might have a registrational plan, but there’s little to suggest that the markets are about to change their view of gamma-delta T cells.
1043













