
ASH 2024 – Jaypirca's confirmation, despite no survival
Crossover scuppers Jaypirca's survival benefit, but full approval seems likely.
Crossover scuppers Jaypirca's survival benefit, but full approval seems likely.

Results just revealed at ASH from Jaypirca's confirmatory trial in BTK inhibitor-relapsed chronic lymphoblastic leukaemia have shown why Lilly waited a whole year from toplining the trial as positive for its primary PFS endpoint before reporting the data in full.
It seems that the company was awaiting the overall survival result, a secondary endpoint of this trial, Bruin CLL-321; that endpoint only matured in August, and it's now been revealed to be a bust. However, the trial's OS analysis was clearly scuppered by 76% of patients who progressed in the control arm opting to cross over to Jaypirca – a result that, for ethical reasons, was unavoidable.
In December 2023 Lilly announced that Bruin CLL-321 met its primary PFS endpoint. This has now been quantified, with ASH data showing a clear PFS advantage for Jaypirca, which nearly halved the risk of progression or death, with a 0.54 hazard ratio versus investigator's choice of Zydelig plus Rituxan or Rituxan plus bendamustine.
Full approval imminent?
As such, Jaypirca looks set to be fully approved for the important CLL indication, a setting in which it received an accelerated greenlight a year ago on the basis of a 72% response rate in the phase 1/2 Bruin study.
As a non-covalent BTK inhibitor Jaypirca is said to remain active where traditional, covalent BTK drugs like Imbruvica or Brukinsa can trigger escape mutations in the BTK protein that cause patients to relapse. The strong Bruin data – also in patients who had relapsed on covalent BTK inhibitors – validated this claim.
However, this efficacy, and the dubious benefit such patients might expect to derive from Zydelig, Rituxan and /or chemo, meant that Bruin CLL-321 had to include the option for control subjects to cross over to active treatment.
Bruin CLL-321 dosed 116 patients with Jaypirca and 109 with the control drugs. 66 patients in the control cohort had investigator-adjudicated disease progression and were thus given the option to cross over to Jaypirca, and 50 of them decided to do so, the ASH presentation revealed.
Such a high rate of crossover, 76%, is almost unprecedented in recent clinical trials, and appears to be rivalled only by 85% crossovers in studies of the radiotherapies Pluvicto and PNT2002. Given the crossover in Bruin CLL-321, median OS came in at 73.4 months for Jaypirca and 70.8 months for control, yielding a highly non-significant hazard ratio of 1.09.
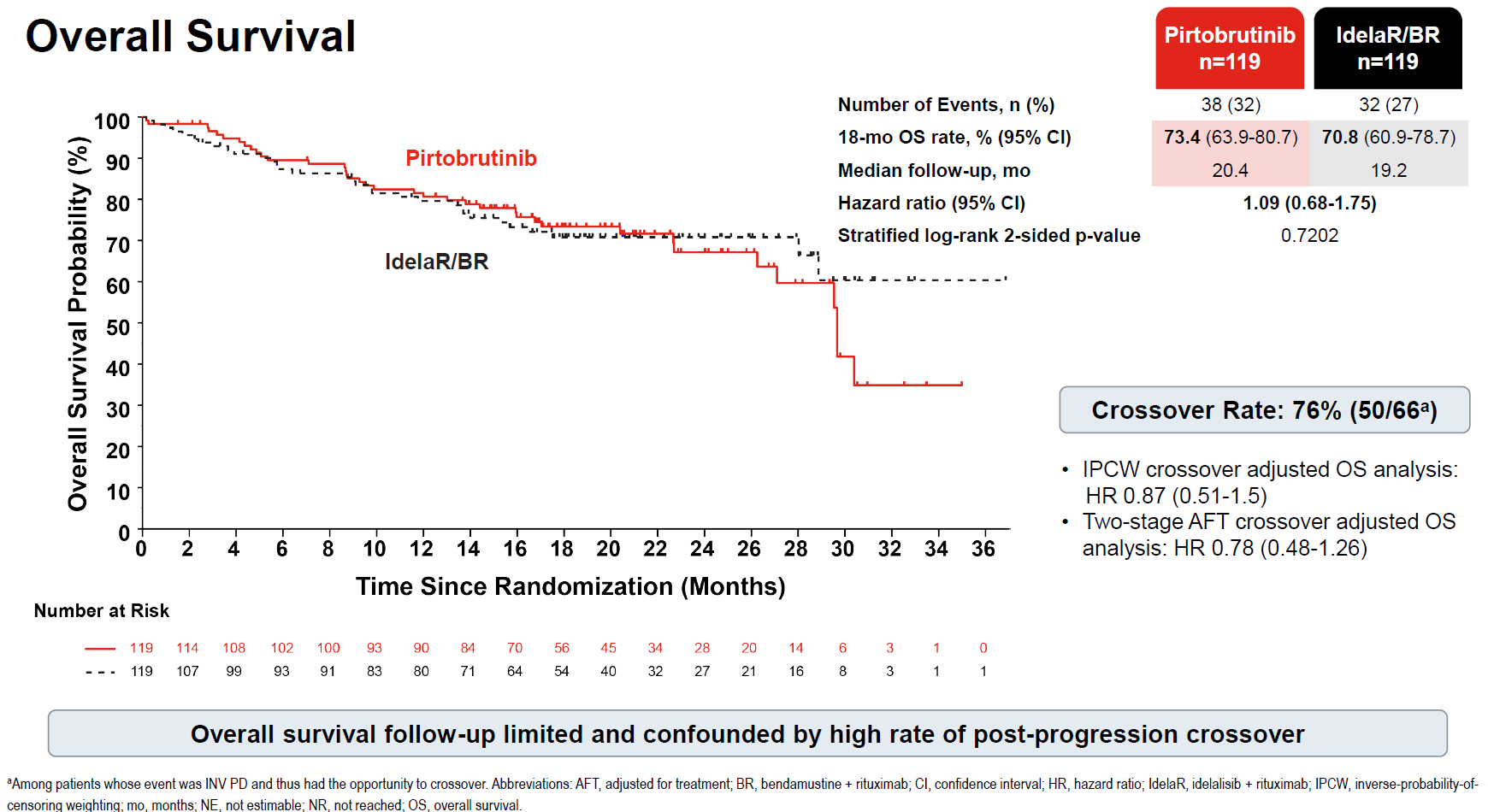
Two different statistical analyses were provided to try and adjust for the effect of crossover, and both indicated hazard ratios that numerically favoured Jaypirca, namely 0.87 and 0.78; however, given the small patient numbers, both came with extremely wide confidence intervals.
The investigators concluded that Japyirca patients were able to delay their next therapy or death for a median of some two years, highlighting the Lilly drug's place in early sequencing. Given how refractory these CLL patients were – all had received a covalent BTK inhibitor, 15% had got two, and 70% had failed on an anti-CD20 therapy, this seems a benefit not to be sneezed at.













