
ASH 2023 – Novartis sees early TIM-3 promise

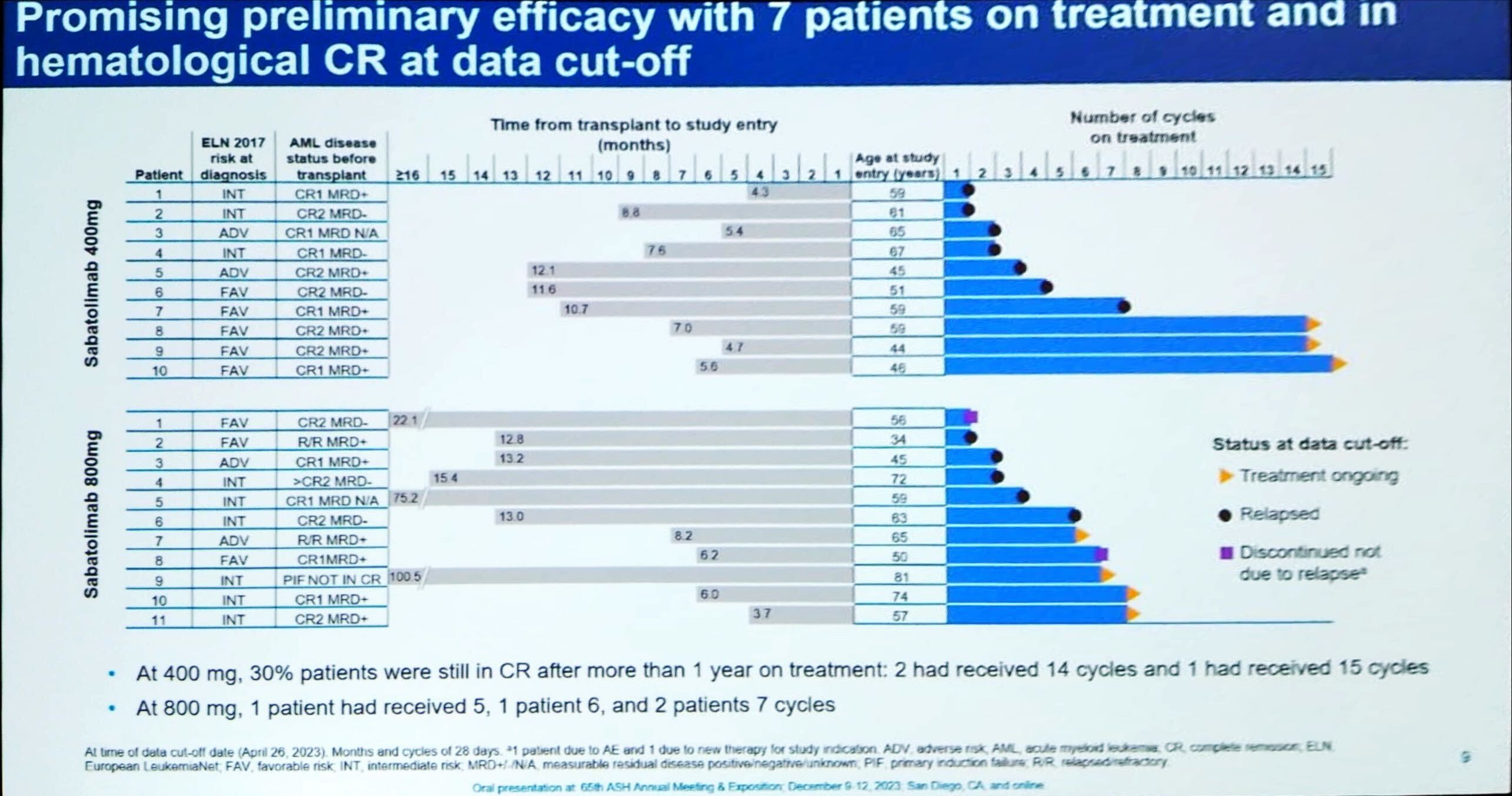
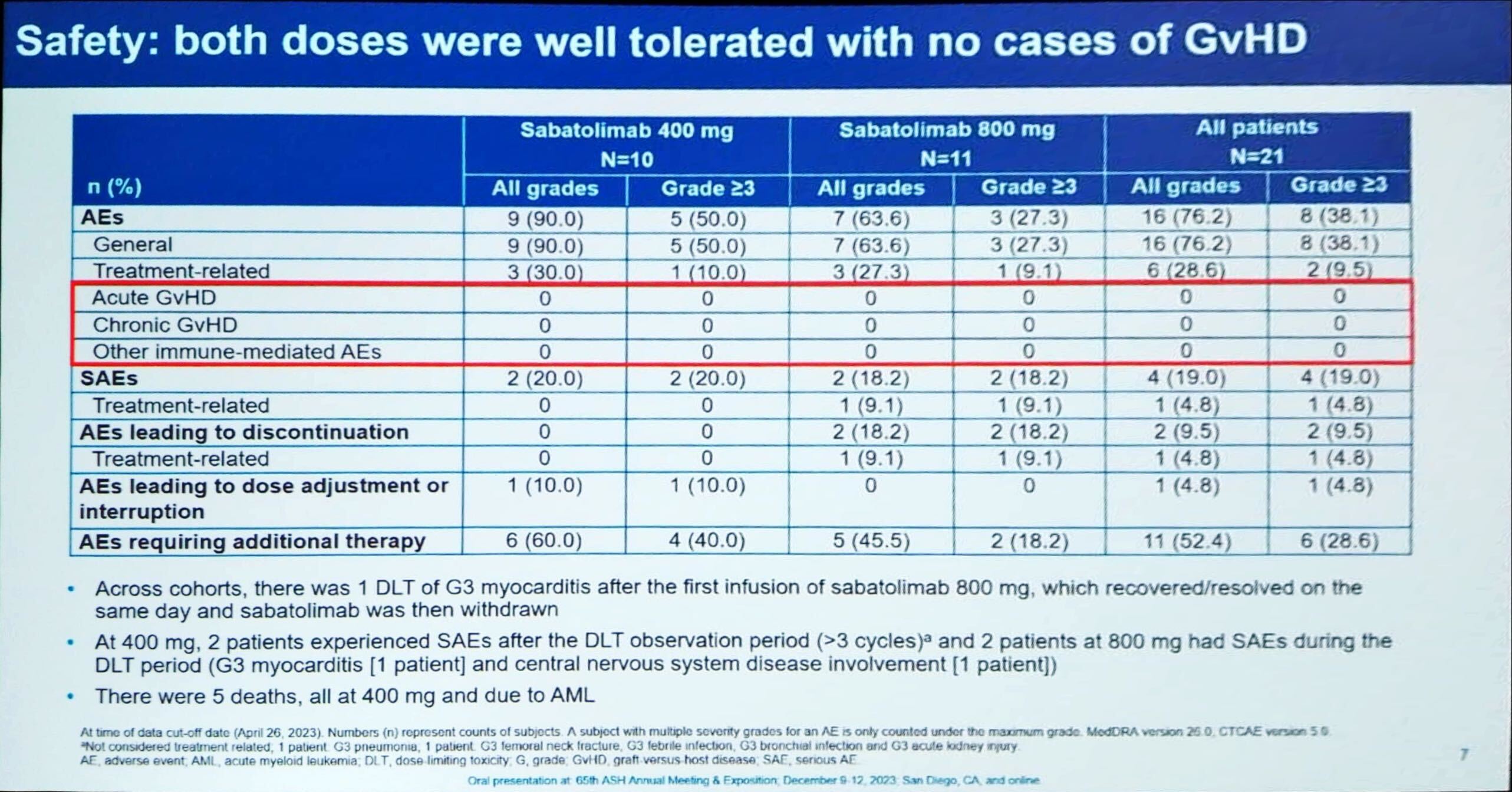
Novartis is leading the TIM-3 inhibitor field, and on Saturday the ASH meeting saw early data with its candidate sabatolimab in a new use, AML maintenance therapy. The results came from the phase 1/2 Stimulus-AML2 trial, with sabatolimab 400mg and 800mg. Presenting the data, Professor Robert Zeiser of the Medical Center University of Freiburg highlighted the fact that 30% of patients on the 400mg dose were in complete response after one year of treatment. He also noted a lack of graft-versus-host disease, which had been an adverse event of interest given that it has been seen with other checkpoint-inhibiting drugs. However, there was one dose-limiting toxicity of grade 3 myocarditis with 800mg sabatolimab; and one patient receiving 400mg had grade 3 neutropenia and thrombocytopenia. An expansion cohort evaluating 800mg with or without azacitidine began enrolling in June. Sabatolimab is also in the phase 2 Stimulus-AML1 trial in first-line unfit patients, with data expected this year, but a phase 3 study will be needed for approval. Novartis’s main TIM-3 rival, GSK, does not appear to be active in AML with its project, cobolimab. But sabatolimab’s most advanced use is myelodysplastic syndrome, where the pivotal Stimulus-MDS2 study will read out next year.
Efficacy with sabatolimab in Stimulus-AML2
Adverse events with sabatolimab in Stimulus-AML2
2128













