
ASH 2023 – Genmab's son of Darzalex in focus

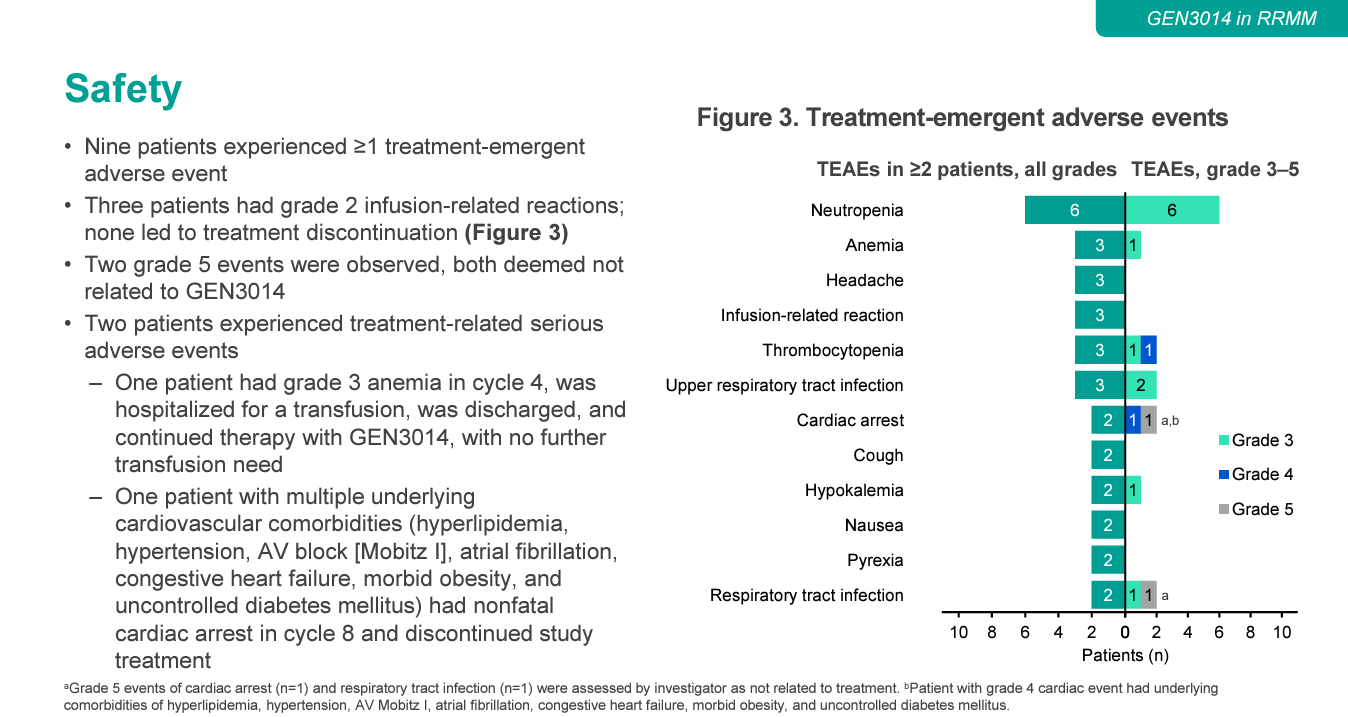
Genmab’s next-generation CD38 project GEN3014 is becoming increasingly important as the company looks beyond its multiple myeloma juggernaut Darzalex. But early data, presented in an ASH poster on Monday, suggest that, while efficacy might be up to scratch, toxicity will be closely watched. An overall response rate of 55% among 11 relapsed/refractory multiple myeloma patients receiving GEN3014 compares well with the 30-40% previously seen with Darzalex monotherapy, Jefferies analysts noted. However, 73% of patients discontinued GEN3014, half because of adverse events. And there were two serious adverse events deemed related to treatment, of anaemia and non-fatal cardiac arrest. The heart attack patient had multiple cardiac comborbidities, so Genmab will hope that this was caused by underlying disease. But there was also a fatal heart attack – although deemed unrelated to GEN3014 – and another unrelated death, from respiratory tract infection. Adverse events will therefore be something to keep an eye on next year, when results are due from the head-to-head portion of this trial, testing GEN3014 against Darzalex. Johnson & Johnson, Genmab’s partner on Darzalex, is expected to make a decision in 2024 on whether to opt in to development of GEN3014.
GEN3014 safety data at ASH 2023
1366













