ASCO Plenary – what Astellas does Keymed might do better
Keymed’s AstraZeneca-partnered anti-Claudin18.2 ADC looks better than Astellas’s zolbetuximab – albeit on an extremely unreliable metric.
Keymed’s AstraZeneca-partnered anti-Claudin18.2 ADC looks better than Astellas’s zolbetuximab – albeit on an extremely unreliable metric.

With Astellas’s zolbetuximab due a US approval decision by 12 January, a low-key project targeting the same antigen, Claudin18.2, is fighting for attention in a crowded field. That asset is Keymed’s CMG901, and it’s notable because its early data were strong enough to secure buy-in from AstraZeneca this year.
Now more results from the same study have been presented at an ASCO Plenary, and on a cross-trial basis they look comparable to zolbetuximab’s registrational chemo combo data – with CMG901 given as a monotherapy and in a later-line setting. That said, Keymed’s data concern remission rates, a metric that has failed to correlate with a survival benefit in either of Astellas’s phase 3 trials.
Zolbetuximab is awaiting approval for first-line, Claudin18.2-expressing gastric/gastroesophageal junction adenocarcinoma. The phase 1 data with CMG901 come from the same cancer type, but the trial uses a different definition of Claudin18.2 positivity, and enrols sicker patients, who had failed a median two lines of prior systemic therapy.
$63m up front
While zolbetuximab is a naked anti-Claudin18.2 MAb, CMG901/AZD0901 is an antibody-drug conjugate. Having earlier picked up rights to Harbour Biomed’s HBM7022, a T-cell engaging anti-Claudin18.2 MAb, Astra in April paid $63m to license CMG901 from Keymed, after seeing promising early data.
At yesterday’s ASCO Plenary these results were updated, with the phase 1 trial’s escalation phase now comprising 89 subjects, given CMG901 at 2.2-3.0mg/kg. The unconfirmed ORR of 44% across all doses, and 61% for 2.2mg.kg, compares favourably versus zolbetuximab’s Spotlight and Glow studies, which benefited from a chemo combo approach and involved less advanced patients.
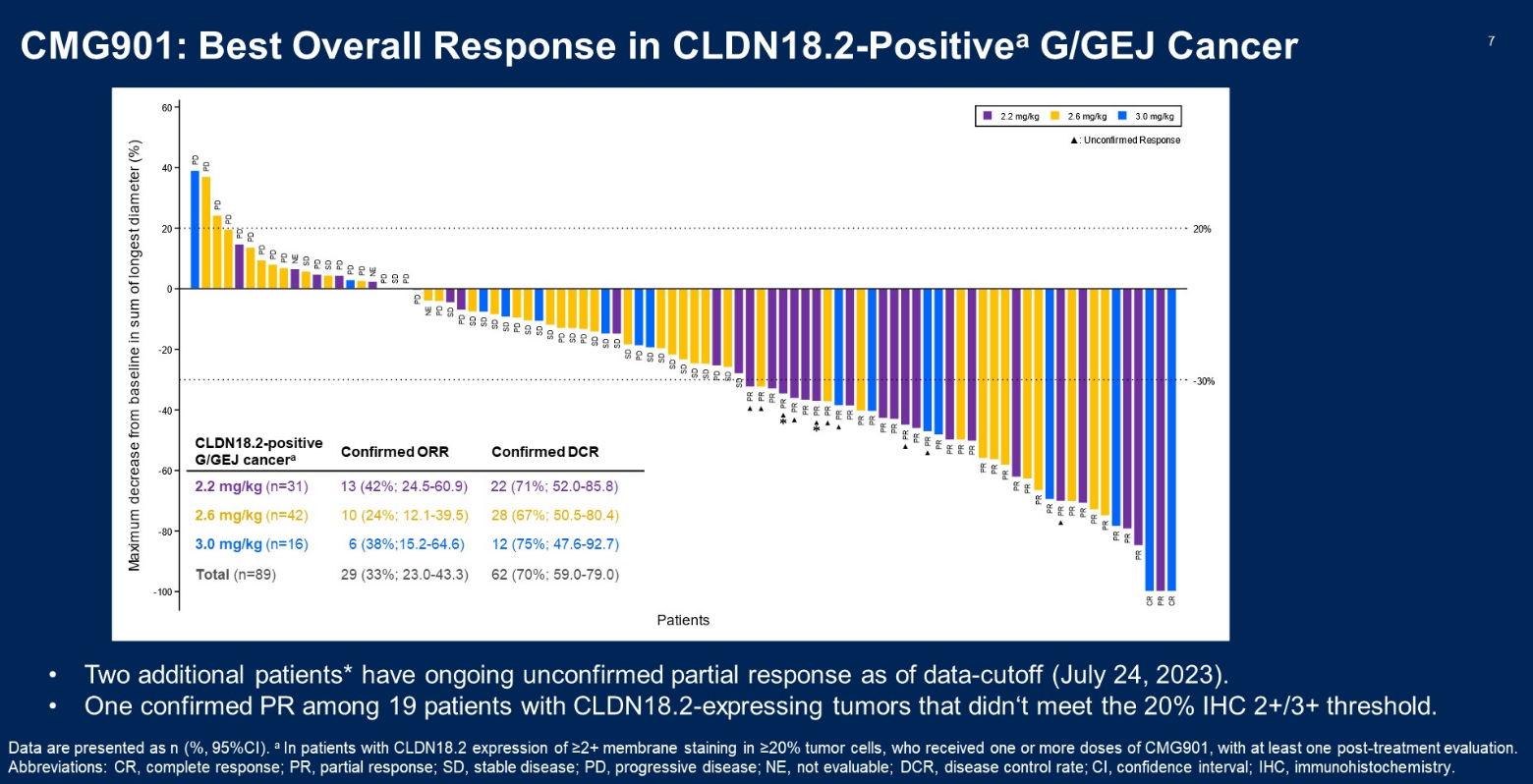
However, an obvious red flag with Keymed’s data is the lack of a dose response; it’s the lowest CMG901 dose that does best, with 3.0mg/kg yielding an ORR of 50%, and 2.6mg/kg just 29%. Similarly, PFS and OS data bore no relation to the dose at which CMG901 was given.
CMG901’s safety profile was described as manageable, though there was one one drug-related death, due cerebral haemorrhage. Meanwhile, vomiting is a Claudin18.2 class effect.
A bigger problem is the fact that zolbetuximab’s pivotal trials failed to show any remission rate benefit versus chemo alone – yet, despite this, in both zolbetuximab showed statistically significant increases in PFS and overall survival. And in the CMG901 trial there’s no correlation either between survival and dose, or between survival and the ORR across the three doses.
Cross-trial comparison of gastric/GEJ adenocarcinoma studies
| Zolbetuximab + chemo (vs chemo) in 1st-line Claudin18.2+ve* disease | CMG901/AZD0901 monotherapy in 2nd to 7th-line Claudin18.2+ve** disease | ||||
|---|---|---|---|---|---|
| Spotlight | Glow | NCT04805307 | |||
| Pts in active cohort | n=283 | n=254 | n=32 | n=45 | n=16 |
| 2.2mg/kg | 2.6mg/kg | 3.0mg/kg | |||
| ORR^ | 60.7% vs 62.1% | 42.5% vs 40.3% | 61.3% | 28.6% | 50.0% |
| CR^ | 5.7% vs 3.3% | 3.5% vs 2.0% | 0.0% | 0.0% | 12.5% |
| PR^ | 55.0% vs 58.8% | 39.0% vs 38.3% | 61.3% | 28.6% | 37.5% |
| mPFS | 10.6mth vs 8.7mth | 8.2mth vs 6.8mth | 4.9mth | 3.3mth | 14.5mth |
| Stats | HR=0.75, p=0.0066 | HR=0.69, p=0.0007 | – | ||
| mOS | 18.2mth vs 15.5mth | 14.4mth vs 12.2mth | NR | 8.5mth | NR |
| Stats | HR=0.75, p=0.0053 | HR=0.77, p=0.0118 | – | ||
Notes: *defined as moderate-to-strong membrane staining in ≥75% of tumour cells by IHC; **defined as expression of ≥2+ intensity in ≥20% tumour cells; ^CMG901 trial includes unconfirmed remissions. Source: Asco.
Nevertheless, the discussant, Dr Elizabeth Smyth of Oxford University, said remission rates for CMG901 appeared better than with current therapies. Second-line chemo, with or without Cyramza, yields ORRs or 7-28%, while the number for third-line chemo is just 4%.
Among the caveats, she highlighted that this was an Asia study, and that gastric cancer demographics are different there; Enhertu, for instance, tends to do better (in Her2-positive settings) in Asia than in the west, even though Asian populations tend to be more heavily pretreated.
She called the PFS seen with CMG901 “fairly average”, but said OS looked good, with the obvious caveat of small patient numbers. Smyth also called attention to the “more permissive” Claudin18.2 expression allowance versus Astellas’s trials; 93 of 113 patients met the criteria for Claudin18.2 expression in Keymed’s study, while Spotlight assessed 2,244 patients, and deemed only 865 of them to be Claudin18.2 moderate to strong expressers.
Though in general around half of gastric cancer patients are thought to express some levels of Claudin18.2, testing is not yet standard practice, but this will change if zolbetuximab comes to market. At least in this regard CMG901 can follow in the slipstream.
2096













