
ASCO-GU – Pfizer eyes broader Talzenna use
But the overall survival benefit in prostate cancer is still driven by HRR mutations.
But the overall survival benefit in prostate cancer is still driven by HRR mutations.

Pfizer’s PARP inhibitor latecomer Talzenna is approved alongside Xtandi for a genetic subtype of metastatic castration-resistant prostate cancer; now the company has its sights on the bigger all-comers market, based on updated results from the first-line Talapro-2 trial.
The data, being presented in a late-breaking session at the ASCO Genitourinary Cancers Symposium, showed an overall survival benefit with Talzenna plus Xtandi, versus Xtandi alone, regardless of homologous recombination repair (HRR) status. However, one of the key investigators has noted that the benefit was driven by HRR-deficient patients, and questioned whether the regimen should be used in men with no HRR alterations.
This appears to confirm fears expressed at the same conference two years ago, and explains the FDA's caution in approving Talzenna plus Xtandi only in the HRR subgroup. Nevertheless, Pfizer now hopes to discuss the Talapro-2 OS data with regulators with the aim of “making the combination available to a broad population of men with mCRPC”, a spokesperson told ApexOnco, without giving details of timings.
HRR-altered
HRR genes are involved in the repair of damaged DNA, and PARP inhibitors’ efficacy is limited to tumours with HRR deficiencies, such as BRCA mutations. HRR deficiencies are said to occur in 20-30% of mCRPC patients, and around 20% of patients in Talapro-2 had this genetic subtype.
The trial prospectively assessed patients for HRR deficiencies and primarily assessed radiographic progression-free survival in both all comers and HRR-deficient patients. Initial results, presented at ASCO-GU 2023, found a 37% reduction in the risk of disease progression or death in all comers, an effect clearly driven by the HRR-deficient subgroup, where the risk reduction was 55%.
At the time overall survival data were immature, and the possibility was raised that final OS data would back only the HRR-deficient subgroup; this is indeed what has come to pass. In June 2023, the FDA approved the combo only in patients with HRR mutations.
Déjà vu?
At this year’s meeting, Pfizer is presenting the final OS results from Talapro-2. In all comers there was a 20% reduction in the risk of death with Talzenna plus Xtandi, with a p value of 0.016.
However, a key analysis – the OS result specifically in HRR-deficient patients – is being revealed at ASCO-GU in a separate poster. Here, Talzenna plus Xtandi reduced the risk of death by 38%, with a p value of 0.0005.
One of the investigators, Dr Karim Fizazi of Institut Gustave Roussy, noted that in patients with no detectable HRR alterations there was "no significant OS benefit". During a pre-ASCO-GU press conference he stated: “You may argue, in this population of men, whether we should use this combination or not.”
Overall survival in patients with no HRR alteration
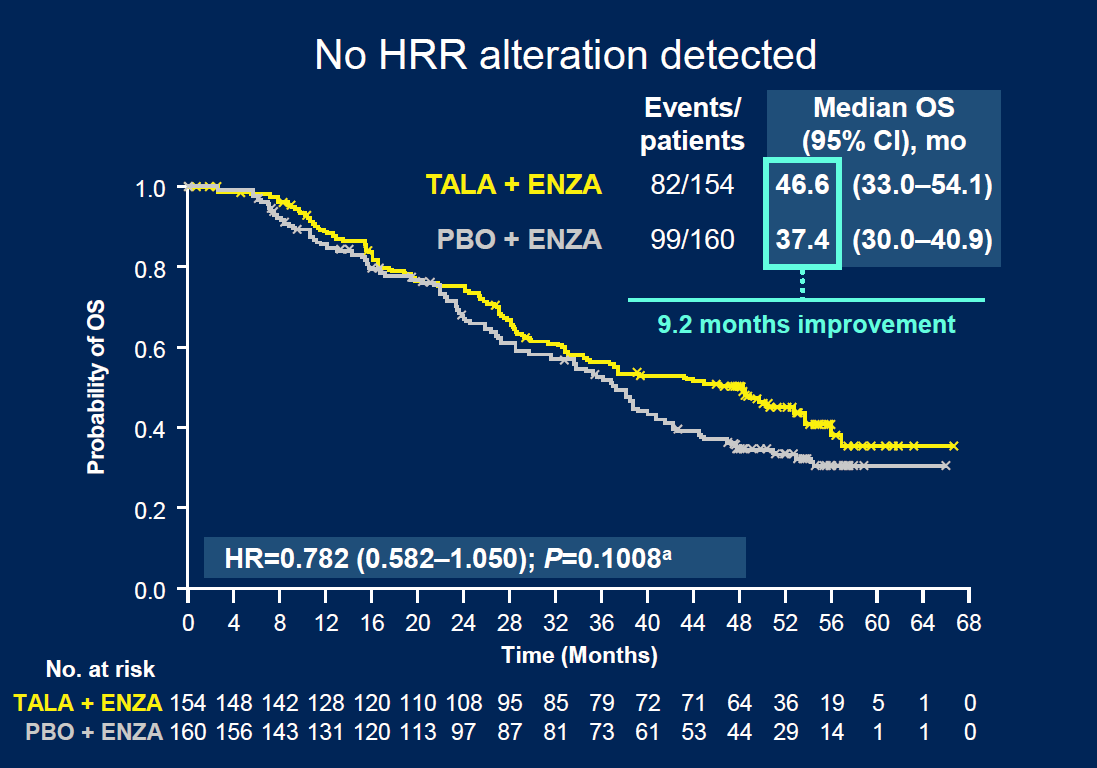
Discussing the data, Dr William Kelly of Thomas Jefferson University Hospital, said the results supported Talzenna plus Xtandi as standard of care for mCRPC, but added that “careful consideration” should be made of the combo’s risk/benefit profile, especially in patients without HRR alterations.
This risks are particularly relevant given the 49% rate of grade 3/4 anaemia with the regimen.
Notably, a similar situation occurred with AstraZeneca/Merck & Co’s PARP inhibitor Lynparza, which in combination with Zytiga produced an all-comers rPFS benefit in its Propel trial, but which improved OS only in HRR-altered patients. Lynparza’s first-line mCRPC approval was limited to BRCA-mutated patients.
Fizazi added that not all HRR-alterations appeared to be equal, with “enormous” benefits in BRCA mutants, but only “modest” improvements seen in those with CHEK2 alterations, for example.
It will soon be up to the FDA to determine what this means for Talzenna’s prostate cancer approval.
Overall survival by subgroup in Talapro-2
| All comers (n=805) | HRR-deficient (n=399) | No HRR alteration (n=314) | ||||
|---|---|---|---|---|---|---|
| Talzenna + Xtandi | Xtandi | Talzenna + Xtandi | Xtandi | Talzenna + Xtandi | Xtandi | |
| OS | 45.8 mth | 37.0 mth | 45.1 mth | 31.1 mth | 46.6 mth | 37.4 mth |
| Stats | HR=0.80 (CI=0.661–0.958); p=0.0155 | HR=0.62 (CI=0.475–0.814); p=0.0005 | HR=0.78 (CI=0.582–1.050); p=0.1008* | |||
Note: *nominal p value. Source: ASCO-GU.
1430













