ASCO-GU – another strike against Cabometyx
A triplet did worse than control in first-line kidney cancer.
A triplet did worse than control in first-line kidney cancer.

Until now the abandonment of a regulatory filing based on the Cosmic-313 study of a Cabometyx/Opdivo/Yervoy triplet in first-line renal cancer was thought to be down to uncompetitive-looking PFS numbers – the study's primary endpoint. Release of full survival data for the first time at ASCO's Genitourinary Cancers symposium showed matters to be worse, however.
Most striking are the negative overall survival numbers, revealed for the first time since Cosmic-313 yielded its technical win on PFS back in 2022; and with longer follow-up the PFS benefit has slipped too. With this triplet no longer part of Exelixis/Bristol Myers Squibb's focus these points are somewhat moot, but they again highlight how difficult it has been to expand Cabometyx's label.
Cabometyx is marketed for renal, liver and thyroid cancers, and first-line renal, as part of an Opdivo combo based on the Checkmate-9ER study, is probably its most important setting. Beyond this there have been numerous failed trials of Cabometyx combinations with checkpoint blockade, though the drug is awaiting a US decision in August on its monotherapy filing for neuroendocrine tumours.
Front-line battle
Cosmic-313 was an attempt to put pressure on Merck & Co/Eisai, whose Keytruda plus Lenvima combo was mounting a strong challenge against Opdivo/Cabometyx in first-line kidney cancer thanks to its registrational Keynote-581 study. Opdivo plus Yervoy is separately approved in this setting (Checkmate-214), hence the logic of adding Cabometyx on top of this doublet.
In mid-2022 Cosmic-313 yielded positive PFS data, with the triplet cutting risk of progression or death by 27% versus the Opdivo/Yervoy doublet. However, it was clear that this was underwhelming: Opdivo/Yervoy itself cuts risk by just 12% versus Sutent, Pfizer’s benchmark renal cancer drug against which Merck’s Keytruda plus Lenvima has shown a 53% reduction in long-term data from Keynote-581.
After that things went quiet, and filing plans were abandoned later that year pending mature OS results, which the FDA had apparently made a condition of a marketing application.
Now those data have shown a median OS benefit of just 41.9 months for Cabometyx/Opdivo/Yervoy, versus 42.0 months for Opdivo/Yervoy, and the hazard ratio numerically favoured control too (1.02, p=0.8). With 46 months of median follow-up the hazard ratio for PFS has slipped from 0.73 to 0.82, ASCO-GU heard.
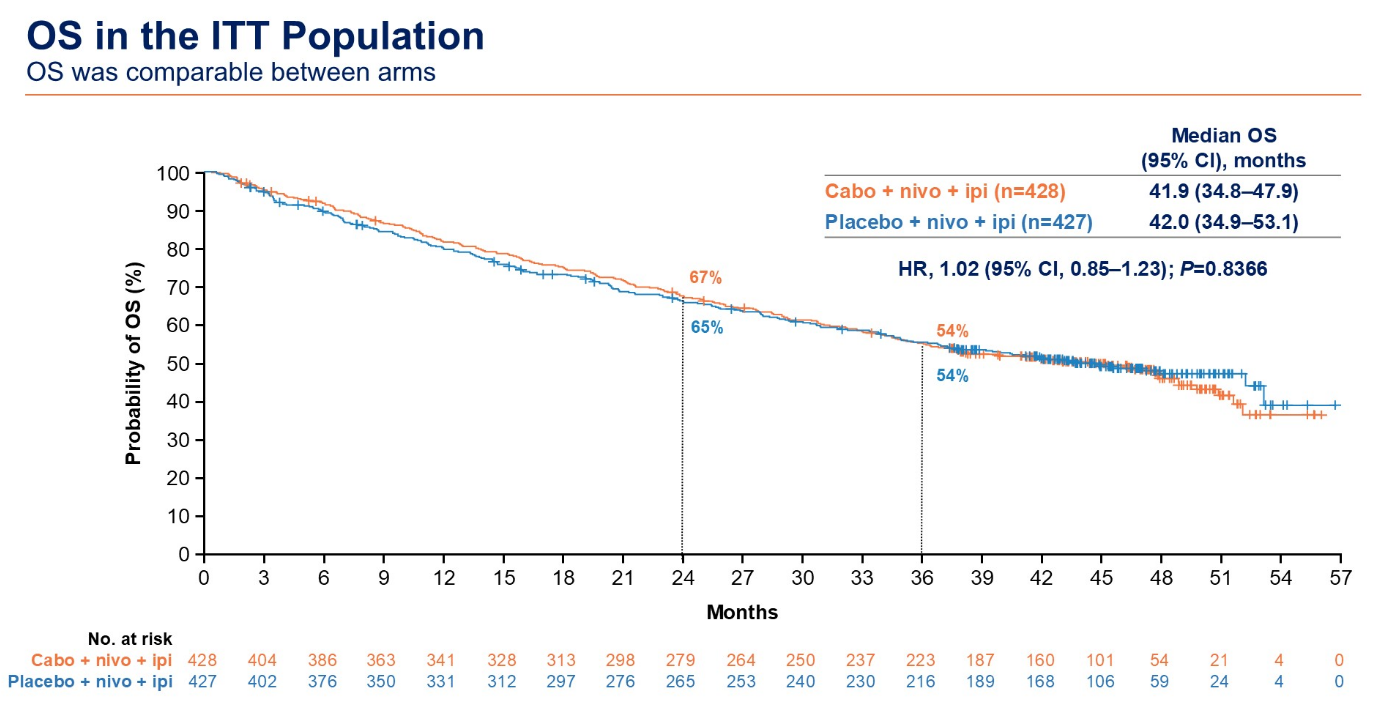
Source: Dr Laurence Albiges & ASCO.
The situation is somewhat reminiscent of another Cabometyx label-extending trial, Contact-02, in combination with Tecentriq in post-hormone therapy metastatic castration-resistant prostate cancer. In 2023 this scored a technical hit on PFS versus hormone switch, but OS was said to be negative at first interim analysis.
Last year ESMO revealed the final OS numbers, with medians favouring control (15.0 months versus 14.8 months for Cabometyx/Tecentriq), and a non-statistically significant hazard ratio of 0.89 (p=0.296). At the same time Exelixis's partner Ipsen said it would no longer pursue filings based on Contact-02 in its territories outside the US and Japan.
For its part Exelixis maintained that a US prostate cancer filing in 2024 remained on the cards, but as of now there has still been no progress on this.
Long-term data in first-line renal cancer
Keytruda + Lenvima | Opdivo + Cabometyx | Opdivo + Yervoy | Opdivo + Yervoy + Cabometyx | |
|---|---|---|---|---|
| Trial | Clear/Keynote-581 | Checkmate-9ER | Checkmate-214* | Cosmic-313* |
| Comparator | Sutent | Sutent | Sutent | Opdivo + Yervoy |
| mPFS | 23.9mth vs 9.2mth | 16.4mth vs 8.3mth | 12.4mth vs 12.3mth | 16.6mth vs 11.2mth |
| HR=0.47 (p<0.0001) | HR=0.58 | HR=0.88 (not stat sig at primary) | HR=0.82** | |
| mOS | 53.7mth vs 54.3mth | 46.5mth vs 35.5mth | 52.7mth vs 37.8mth | 41.9mth vs 42.0mth |
| HR=0.79 (p=0.0424) | HR=0.79 | HR=0.72 (stat sig at primary) | HR=1.02 (p=0.8366) |
Notes: *in intermediate/poor-prognosis patients; **HR was 0.73 (p=0.01) at first interim analysis. Source: OncologyPipeline.
900













