ASCO-GI – confirmation for Bristol’s combo, but is there more?
Opdivo plus Yervoy scores a remarkable survival benefit in Checkmate-8HW, whose control arm presents some comparability difficulties.
Opdivo plus Yervoy scores a remarkable survival benefit in Checkmate-8HW, whose control arm presents some comparability difficulties.
Checkmate-8HW is the first phase 3 study showing that Opdivo plus Yervoy is better than chemo in MSI-high/MMR-deficient colorectal cancer, ASCO’s Gastrointestinal Cancers Symposium heard today. But is the combo better than Keytruda?
That’s the question doctors will want answered, since the Merck & Co drug is the only anti-PD-(L)1 MAb that at present boasts a first-line MSI-H/dMMR colorectal cancer label, and thus represents an important treatment option. Checkmate-8HW didn’t include a Keytruda cohort, so cross-comparison versus Merck’s registrational Keynote-177 trial is necessary.
Even this is complicated, since median PFS hasn’t been reached in Checkmate-8HW’s combo cohort in first-line patients. However, it’s hard to ignore the survival curves unveiled at ASCO-GI by Sorbonne Université’s Dr Thierry André, showing a remarkable separation, starting at about two months, in favour of the Opdivo plus Yervoy doublet.
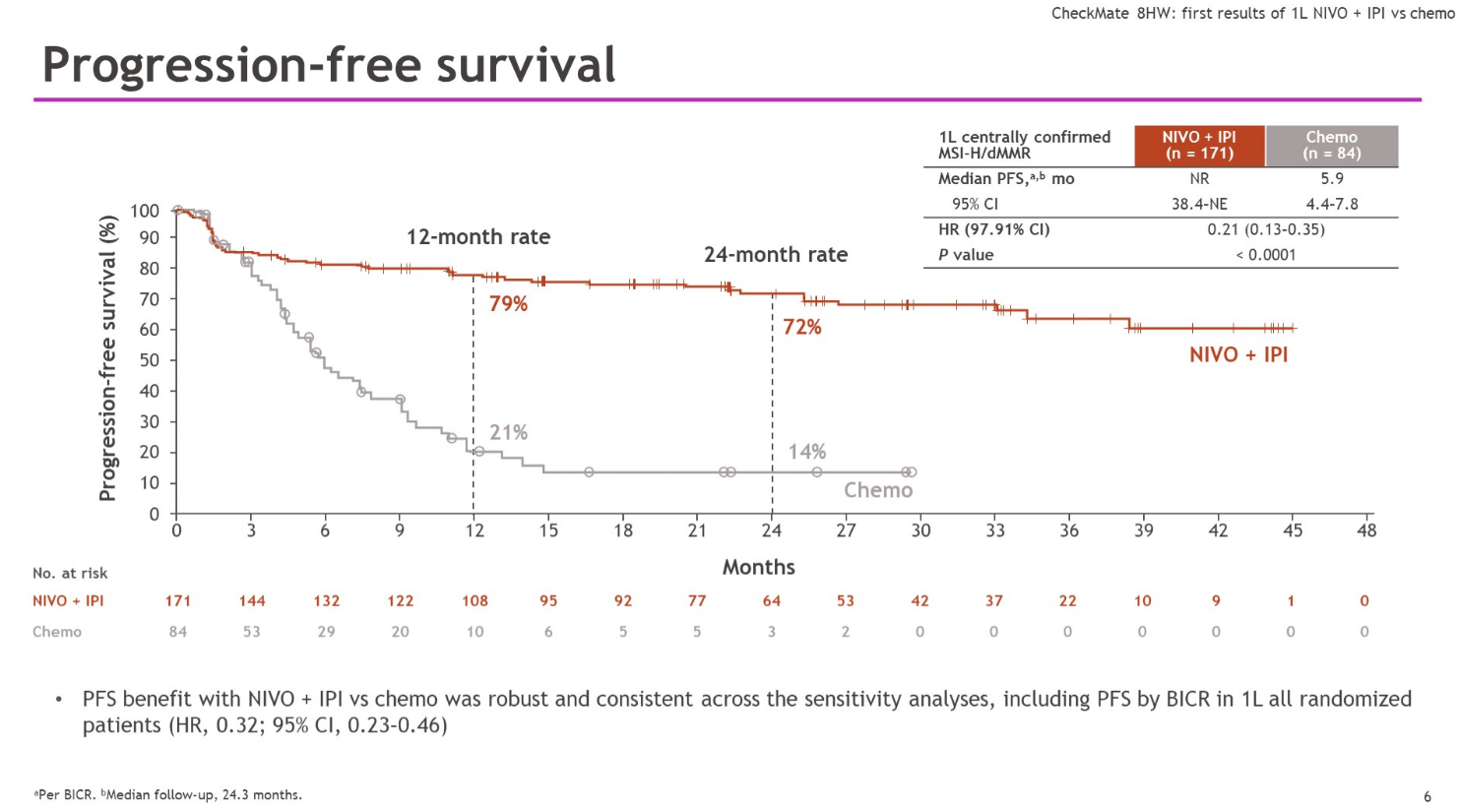
This has translated into an astonishing 79% reduction in risk of progression or death in the Bristol study – the corresponding number in Keynote-177 is 40%. André called Opdivo plus Yervoy a new first-line standard-of-care option.
One caveat is that, on a cross-trial basis, the control cohort in Checkmate-8HW has underperformed that in Keynote-177. Checkmate-8HW also included a third cohort, comprising pretreated patients given Opdivo alone. Opdivo with or without Yervoy already has a second-line MSI-H/dMMR colorectal cancer label, but nothing has been disclosed about the monotherapy cohort in Checkmate-8HW.
Further notable details revealed about Checkmate-8HW include that the PFS benefit held up in BRAF and KRAS/NRAS mutant disease, along with all key subgroup analyses presented. Serious treatment-related adverse event rates were equal across the two cohorts, though both treatment-related deaths, one from myocarditis and the other from pneumonitis, occurred on Opdivo plus Yervoy.
While Checkmate-8HW is continuing towards assessment of the key secondary of overall survival, it appears that PFS alone might be an approvable endpoint in this setting: it’s PFS alone that backs Keytruda’s first-line approval, since Keynote-177 yielded a statistically negative result for overall survival.
Opdivo/Yervoy’s second-line MSI-H/dMMR colorectal cancer nod, backed by the Checkmate-142 trial, was given on an accelerated basis, and at the very least Checkmate-8HW looks like a positive confirmatory trial. Bristol will be hoping for much more.
Cross-trial comparison in 1st-line MSI-H/dMMR colorectal cancer
| Trial | Active cohort | Control |
|---|---|---|
| Keynote-177* | Keytruda | Chemo +/- Avastin or Erbitux |
| mPFS (mth) | 16.5 | 8.2 |
| Stats | HR=0.60, p=0.0004 | |
| Checkmate-8HW** | Opdivo + Yervoy | Chemo +/- Avastin or Erbitux |
| mPFS (mth) | NR | 5.8 |
| Stats | HR=0.21, p<0.0001 | |
Notes: *final OS analysis yielded HR=0.74, which failed to clear a 0.0246 significance boundary; **Checkmate-8HW also included pretreated patients, as well as an Opdivo monotherapy cohort, but neither of these analyses has been reported. Source: ASCO & prescribing information.
This is an updated version of a story published earlier.
1428













