
ASCO 2024 – Scemblix could set new standard in front-line leukaemia
But will the FDA accept the surrogate endpoint used in ASC4First?
But will the FDA accept the surrogate endpoint used in ASC4First?

Novartis’s franchise-protection efforts in chronic myeloid leukemia are now complete, with the group’s next-generation product Scemblix set to take over from its ageing stalwart Gleevec in first-line disease. The ASCO meeting heard today of Scemblix’s victory over Gleevec – and other TKIs – in the ASC4First trial, with the investigator describing the results as practice changing.
The question now is whether regulators will accept ASC4First's surrogate endpoint of major molecular response (MMR), rather than survival. Novartis told ApexOnco that it had filed the results via the real-time oncology review programme, but news of an FDA acceptance has not emerged.
The company added that the most recent approvals in first-line CML were based on MMR, and cited FDA guidance positioning this as a relevant clinical endpoint in CML, predictive of long-term outcomes.
Discussing the results at a press briefing ahead of ASCO, Dr Oreofe Odejide of Dana-Farber Cancer Institute noted that it was difficult to show an overall survival benefit in a chronic disease like CML.
Dr Jorge Cortes of the University of Texas MD Anderson Cancer Center, who presented the data, also defended the use of MMR, saying this captured other benefits beyond survival that have become important as treatment of CML has evolved. Notably, MMR is a good predictor of sustained molecular responses that could make patients eligible for treatment discontinuation, he said.
Scemblix's current accelerated approval, in third-line CML, is based on MMR, so there's already a precedent for this surrogate endpoint. Even if this type of accelerated approval is possible in the first-line setting, the FDA would likely need confirmatory survival data.
Safer and more efficacious
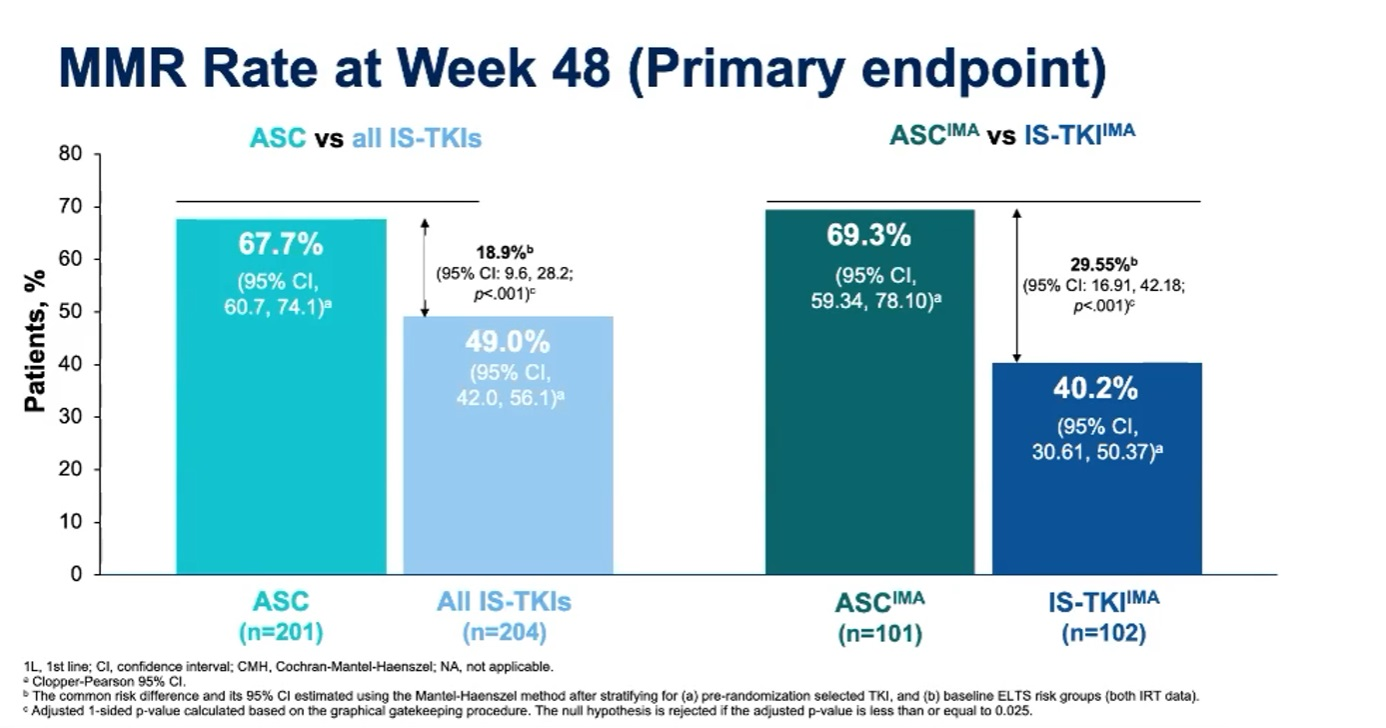
These questions aside, the ASC4First data looked emphatic. The study randomised 405 patients to either Scemblix or physician’s choice of TKI – half of the patients in the TKI arm received Gleevec, while the other half got a second-generation drug such as Tasigna, Sprycel or Bosulif.
The co-primary endpoints were MMR at week 48 for Scemblix versus all TKIs, and versus Gleevec alone. 19% more patients receiving Scemblix achieved MMR at this time point versus all TKIs, and 30% more Scemblix patients reached this threshold versus Gleevec.
Another important factor in a setting like CML, in which patients are often on drugs for many years, is tolerability. Here Scemblix looked good too, showing an “equivalent or better” adverse-event profile versus the established drugs, according to Cortes.
Treatment-related discontinuation rates were also lower with Scemblix (5%) than the other TKIs (11%) and Gleevec (10%).
“It’s good to see we have a good balance of both of these benefits with Scemblix,” Cortes concluded, although he added that the data were early.
PFS and OS are both secondary endpoints of ASC4First, but are listed low down in the hierarchy, according to clinicaltrials.gov.
Novartis’s goal of making Scemblix a multibillion dollar therapy will depend on it moving out of its current late-line CML setting and into treatment-naive patients. Over to the FDA.
1717













