
ASCO 2024 – new matching strategy delays Caribou
The company’s pivotal trial is now set to start in the second half of 2025, from year-end 2024 previously.
The company’s pivotal trial is now set to start in the second half of 2025, from year-end 2024 previously.

Caribou Biosciences had hoped that its lead project, CB-010, could avoid the relapses that have hit other allogeneic Car-T contenders, but data released today, along with a change in strategy, have thrown doubt on this ambition. The group is now focusing on human leukocyte antigen (HLA) matching between donor cells and patients in a bid to improve persistence – a pivot that will delay the start of a planned pivotal trial by at least six months.
The company’s chief executive, Rachel Haurwitz, insisted that the results, which will be presented in a poster at ASCO tomorrow, still support Caribou’s aim of having an anti-CD19 allogeneic Car-T that could rival currently approved autologous products. But, with the company’s stock at an all-time low even before this latest update, investors have yet to be convinced.
HLA matching
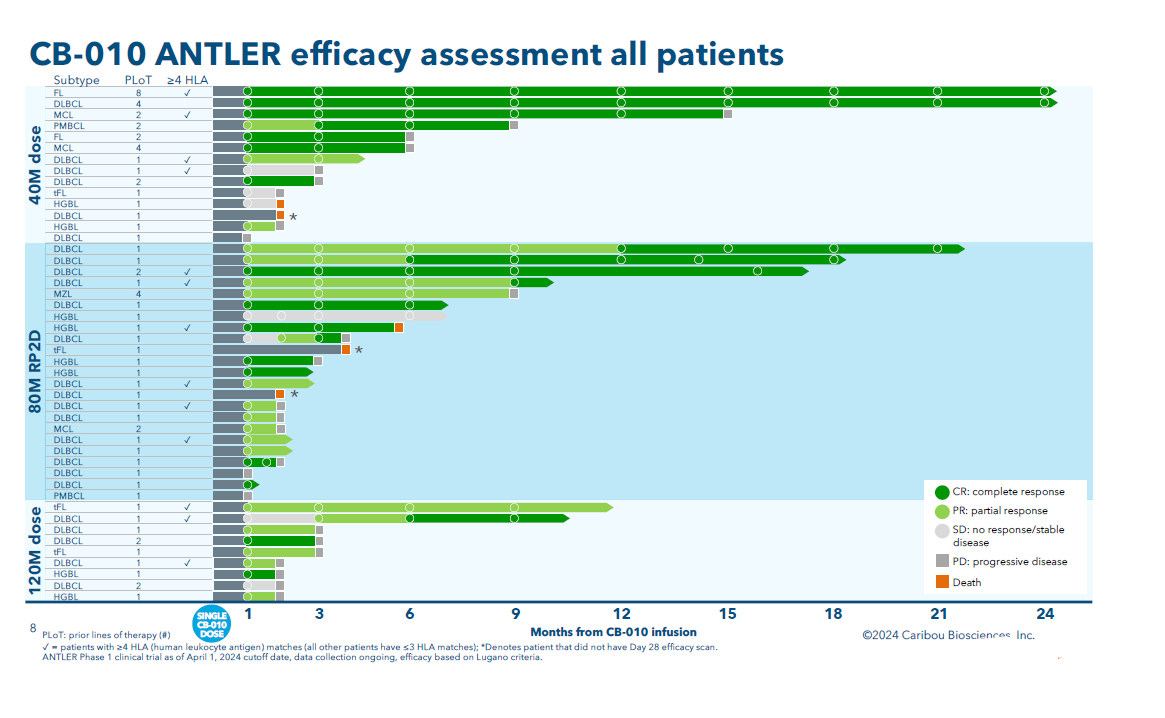
The latest results come from the uncontrolled phase 1 Antler trial in second-line lymphoma, which tested CB-010 dosed at 40, 80 and 120 million Car-T cells. Data from 16 dose-escalation patients were reported last year, and today’s update also includes 30 patients in the dose-expansion portion.
The new data show a waning in response rates versus the previous update, but perhaps more worrying was an overall median duration of response of just five months and a six-month PFS rate of 35%.
The evolving Antler dataset
| ASCO 2024 | Jul 2023 update | |
|---|---|---|
| Cutoff | 1 Apr 2024 | 20 Jun 2023 |
| N | 46 | 16 |
| ORR | 76% | 94% |
| CR rate | 46% | 69% |
Source: ASCO 2024 & OncologyPipeline.
Results were slightly better – but not much – in patients treated with the now-selected go-forward dose, 80 million Car-T cells.
But it is HLA matching, not dose, that Caribou thinks will make all the difference, and it has presented a retrospective analysis that, it believes, backs this up.
HLA is a marker used by the body’s immune system to ascertain what is self and what is foreign. Caribou’s thinking is that the better the match the longer it will take before CB-010 is cleared by a patient’s immune system, and the more activity the product will therefore have, Haurwitz explained during an interview at ASCO.
Among 13 patients with four or more matching HLA alleles, PFS was 14.4 months, versus 2.8 months in patients with three or fewer matches.
Caribou now needs to prove its matching theory prospectively; to this end, the company plans to enrol another 20 second-line large B-cell lymphoma (LBCL) patients who have at least four matched alleles. Data from these patients are due in the first half of 2025 and Caribou wants to have these results before it begins its pivotal trial, hence the delay.
Commercially, HLA matching seems to suggest that 13 different batches of CB-010 would need to be available off the shelf, to be able to provide a partially matched HLA product to around 90% of lymphoma patients.
Pivotal plans
As for its phase 3 plans, Caribou previously said the FDA had agreed to a trial comparing CB-010 against platinum/chemotherapy and autologous stem cell transplantation, rather than against an autologous Car-T like Gilead’s Yescarta.
When asked if anything had changed here, Haurwitz would only say that Caribou owes a visit to the FDA.
She did add that, assuming the HLA-matched data hold up in the next 20 patients, the group expects to use a “best match” strategy in phase 3 to cater to the 90% of patients; the remaining 10% would be matched as closely as possible. This means patients won’t be excluded from the pivotal study based on HLA type.
Still, this pivotal trial is now a long way away. Caribou’s remaining investors will need to keep the faith for a little longer.
2704













