
ASCO 2023 – Morpheus spurs Roche to take the red pill
After generating unexpectedly positive TIGIT data Roche moves to launch a phase 3 trial.
After generating unexpectedly positive TIGIT data Roche moves to launch a phase 3 trial.

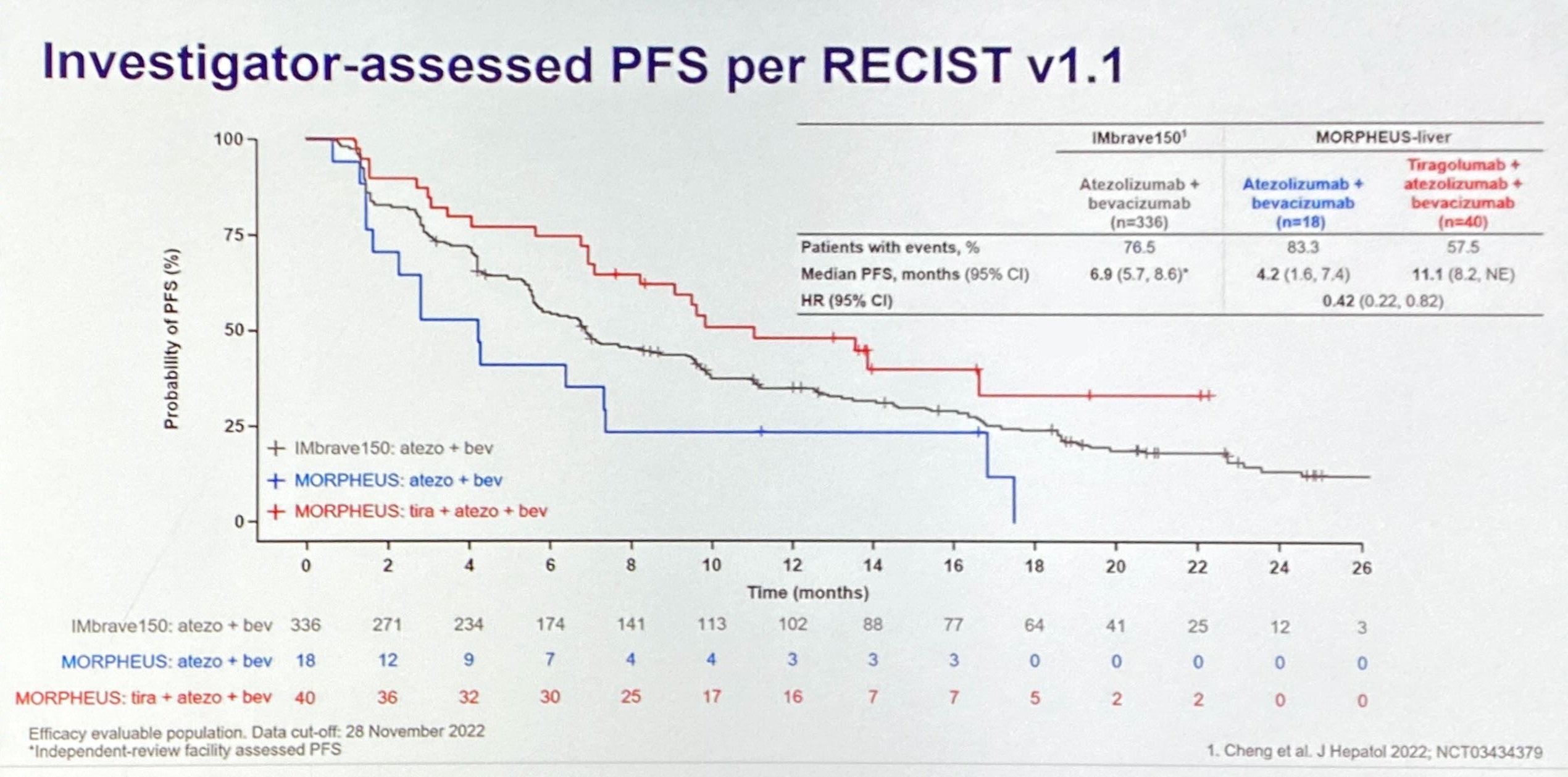
Yesterday more air escaped the TIGIT bubble, thanks to Arcus/Gilead’s deteriorating dataset, but today ASCO brought a different bombshell. Roche’s fact-finding mid-stage Morpheus-liver trial, which had enthused some TIGIT-focused investors a week ago, has delivered a strong enough signal for a phase 3 study to be launched, Asco learned this afternoon.
UCLA’s Dr Richard Finn, who presented the Morpheus-liver data in full, outlined a global, placebo-controlled study, Imbrave-152/Skyscraper-14, that would compare tiragolumab, Tecentriq plus Avastin versus a Tecentriq/Avastin doublet in the front-line setting. This, he told ASCO, would measure overall and investigator-assessed progression-free survival as co-primary endpoints, and was “expected to begin soon”.
His enthusiasm was backed not only by the topline PFS numbers generated by Morpheus-liver, but also by a retrospective analysis using “Bayesian dynamic borrowing” designed to generate possible “synthetic” control arms. The purpose of this was to counter one of the readout’s biggest criticisms, namely that Morpheus-liver’s actual control arm vastly underperformed the performance of Tecentriq plus Avastin in Roche’s Imbrave-150 trial, flattering the result.
The new analysis aimed to counteract imbalances in patients’ baseline criteria, and generated three possible more realistic controls, ranging from a conservative to a fully matched scenario. Each generated a positive reduction in risk of progression or death, from 28% to 51% with tiragolumab, Tecentriq plus Avastin, versus a Tecentriq/Avastin doublet. Meanwhile, Morpheus-liver showed a 58% reduction.
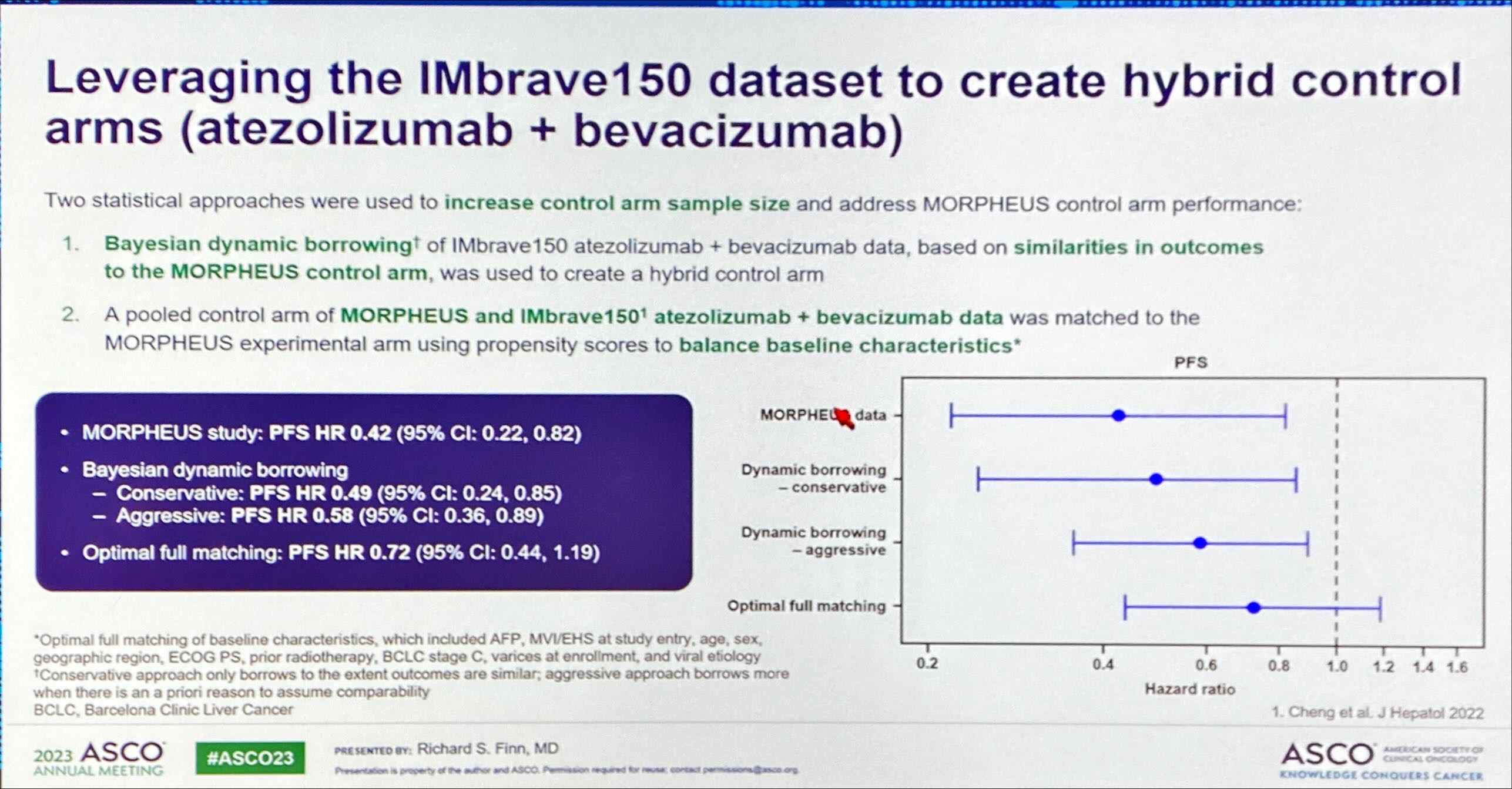
That said, it’s not entirely clear why the mid-stage trial’s actual control cohort underperformed. The ASCO presentation revealed patients’ baseline imbalances, some of which favoured active treatment, though others, including disease severity, favoured control, and Morpheus-liver overall contained a lower percentage of hepatitis B patients than Imbrave-150.
All these considerations aside, Finn said the fundamental confounding issue might have been Morpheus-liver’s relatively small size. The study discussant, Memorial Sloan Kettering’s Dr Ghassan Abou-Alfa, said that while Bayesian modelling was intriguing Morpheus-liver should simply be celebrated for delivering a positive result that now needed corroborating in a larger trial.
682













