
Aegean in the clear
But Imfinzi's precise setting is uncertain, and big problems loom for companies pursuing similar trials.
But Imfinzi's precise setting is uncertain, and big problems loom for companies pursuing similar trials.

Based on the innocuous discussion of AstraZeneca’s Aegean trial at yesterday’s US advisory panel Imfinzi looks headed for US approval in the lung cancer indication in question. The panel wasn’t asked to vote for or against, but the tone of the debate, and the lack of sharp criticism, were notable.
What was put to the vote was whether new studies that, like Aegean, concern perioperative settings should use a more complex design to spell out individual contributions of treatment stages, and here the panel was adamant, by 11 votes to none, that they should. This means more patients, greater expense, and longer time to readout, as Astra laid out in its defence of Aegean.
As adcom briefing documents made clear, the problem with Aegean wasn’t its (positive) results, but the fact that it was impossible to discern the neoadjuvant and adjuvant stages' contributions to the overall result. In Aegean Imfinzi plus chemo was given before NSCLC surgery, and then Imfinzi monotherapy afterwards, but only the combined effect was compared against chemo alone.
In its defence Astra argued that a four-arm trial of the type reviewers suggested was “optimal” would have required about 2,500 patients to be enrolled, instead of 800, and taken 10-12 years to report results. Such issues now become a problem for future applicants, and the FDA noted that about 20 studies of similar “perioperative” settings used simple two-arm designs.
Critical
There were few critical comments during the adcom, but perhaps the sharpest came from Richard Pazdur, director of the FDA's Oncology Center of Excellence, during a question-and-answer session.
He asked for the adcom’s support in curbing future trials that added adjuvant on top of neoadjuvant therapy in a two-arm design, and threatened: “We could ... put these studies on hold. That’s a very draconian action but that may be necessary.”
FDA experts were asked why issues were now being raised when last October Merck & Co’s Keytruda was approved, in a similar perioperative NSCLC setting on the back of the two-arm Keynote-671 trial. An agency speaker accepted that ‘671 was “the elephant in the room”, but claimed that its design drawbacks were “discussed heavily” at the time of the approval.
“We are not a victim of our past action,” Pazdur added. “That was then, and this is now.” Voting members evidently took the same view as regards Aegean, one stating that that study was set on its path six years ago, so its relevance should stand.
Another highly relevant trial here is Bristol Myers Squibb’s Checkmate-77T, testing neoadjuvant Opdivo plus chemo followed by Opdivo monotherapy in NSCLC. Because this is under review the FDA wouldn’t comment on it at the adcom.
What setting?
If Astra’s Aegean-based filing is now in the clear, a remaining question is in what precise setting the FDA will approve Imfinzi.
There was confusion over this, with Astra telling yesterday’s adcom that the proposed indication was neoadjuvant chemo combo followed by Imfinzi monotherapy after surgery – a full perioperative label. However, that same morning Astra’s second-quarter investor deck listed, among upcoming regulatory decisions, “Imfinzi – NSCLC (neoadjuvant)”.
One clue is that the Aegean regimen is already approved in the UK and Switzerland, a little-noticed fact that was stated at the adcom. These approvals appear to concern the full perioperative setting, which might be surprising given Imfinzi’s repeated phase 3 failures specifically in adjuvant NSCLC.
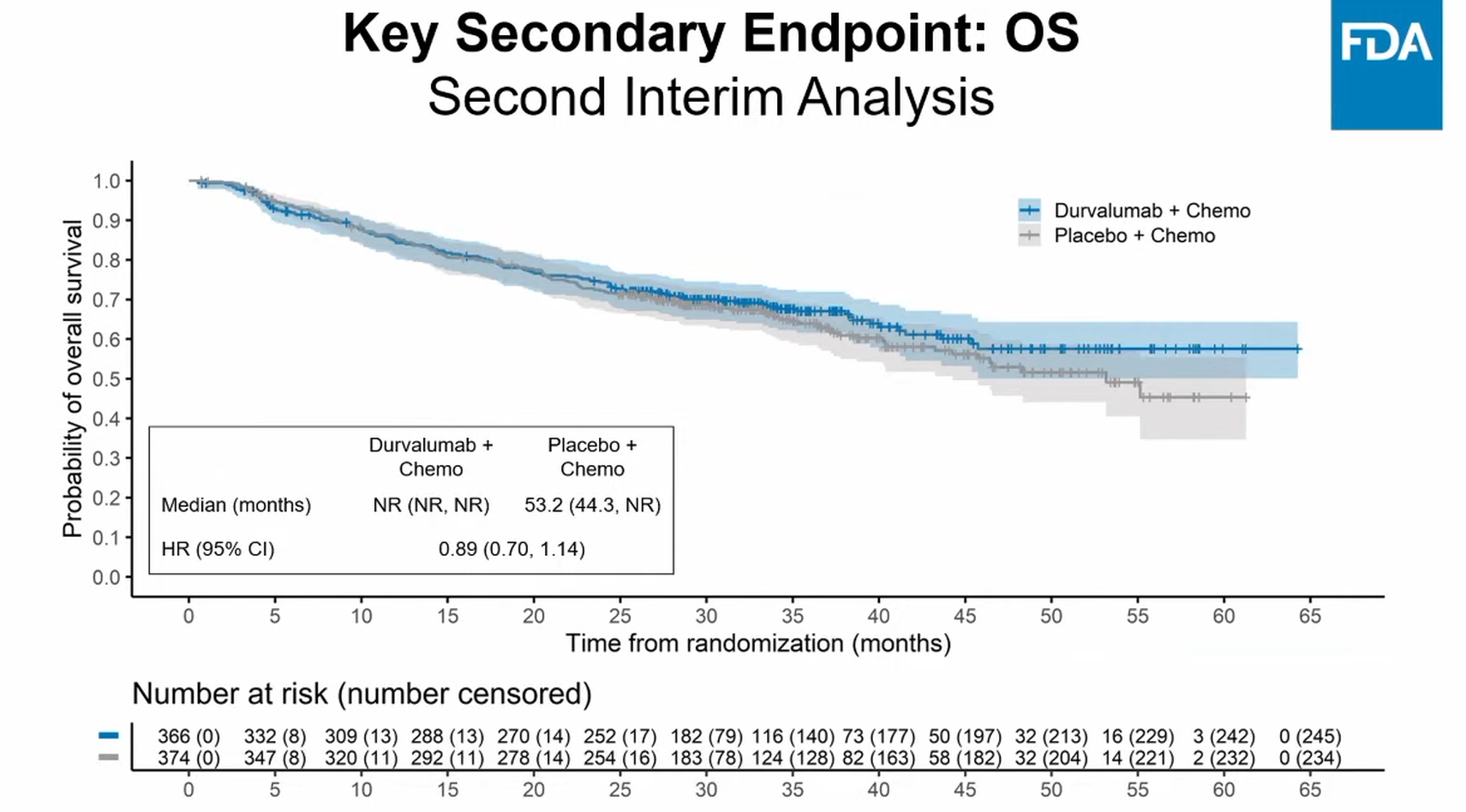
The adcom also revealed an early cut of survival data from Aegean – possibly relevant since Keynote-671 has already read out positively for OS. Aegean’s survival curves, from a 10 May 2024 cutoff at which around a third of the study’s participants had died, showed a reduction in risk of death of 11% favouring the Imfinzi regimens.
Still, even if a statistically significant OS benefit is eventually shown this won’t address the fact that such trials are incapable of discerning their neoadjuvant and adjuvant phases’ contributions, the adcom heard.
Overall survival in Aegean
1317













