
AACR 2025 – Merck's head and neck headscratcher
Keynote-689 delivers a win in all-comers, but how did PD-L1-negatives do?
Keynote-689 delivers a win in all-comers, but how did PD-L1-negatives do?

In filing Keytruda for the perioperative treatment of head and neck cancer Merck & Co has given the FDA a double headache: should the agency approve the drug without knowing how much benefit accrues in the neoadjuvant and how much in the adjuvant phase, and should a label be all-encompassing or limited to PD-L1 expressers?
By the 23 June PDUFA date the FDA will have given its verdict, but for now the supporting trial, Keynote-689, has been presented for scrutiny at an AACR plenary. This has shown positive and statistically significant EFS numbers for PD-L1-expressing patients and all-comers alike, but with no adjacent data provided it's impossible to tell how PD-L1 non-expressers fared.
It is evident from extrapolation, however, that PD-L1 expressers helped drive the benefit in broader patient groups, and it will be up to the FDA whether to issue a similarly broad approval.
Last October Merck said this trial, of neoadjuvant Keytruda followed by adjuvant Keytruda plus chemo and radiotherapy, met its primary endpoint of EFS (event-free survival) versus chemo/radiotherapy alone.
Keynote-689 used a sequential analysis plan that looked first at PD-L1 ≥10%, then at ≥1% expressers, and finally at all-comers. This yielded progressively worsening, though statistically significant, hazard ratios – 0.66, 0.70 and 0.73 respectively – illustrating the deleterious effect on the overall data of including more patients with lower levels of PD-L1 expression.
The EFS readouts applied a one-sided p value of 0.025 as the boundary to be cleared, and thanks to sequential analysis this was preserved for all prespecified data cuts, as long as the previous analysis was positive. At this analysis all three EFS cuts yielded statistically significant p values, of 0.0022, 0.0014 and 0.0041 respectively, AACR heard.
No OS, no problem?
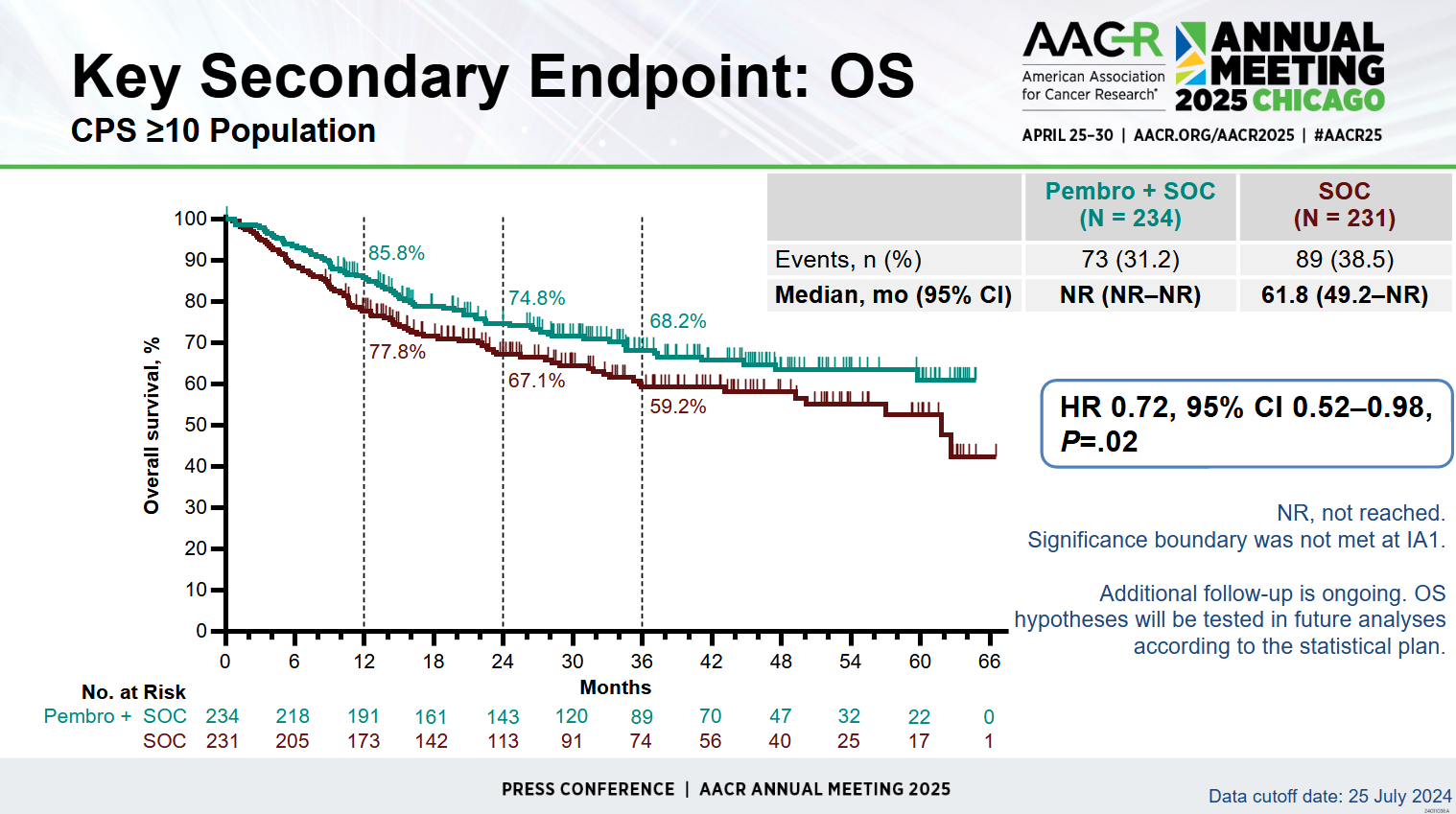
The next stage in Keynote-689's sequential analysis tested major pathological response, which was also positive, before overall survival was analysed. Here Merck already revealed previously that at 81% maturity no benefit was seen in PD-L1 ≥10%-expressers, the first subgroup tested, and as a result no further OS analysis took place.
This might have served as a black mark against Keytruda's use in this setting, but at AACR the OS numbers were revealed, showing a very near miss on this key secondary endpoint in the first subgroup. Not only do the (heavily censored) survival curves separate early and stay separate, they yield a 0.72 hazard ratio with confidence interval upper bound below 1.00, and a p value of 0.02.
It seems unlikely that such numbers will trouble the FDA unduly. Of more concern might be the fact that Keynote-689 included both the neoadjuvant and adjuvant phases, without splitting out Keytruda's relative contribution to each one – something the agency has indicated that it wants to see in perioperative studies.
However, judging by recent approvals of AstraZeneca's Imfinzi based on the Aegean and Niagara trials, and of Bristol Myers Squibb's Opdivo based on Checkmate-77T, such a stance apparently applies only to newly initiated studies and not retrospectively to those that, like Keynote-689, began years ago.
91













